Anti-Histamine (Medicinal Chemistry)
Antihistamines
(Medicinal Chemistry)
Antihistamines
Histamine, chemically known as 2-(4-imidazolyl)-ethyl amine, is an autacoid (which means it acts similarly to a local
hormone, near its site of synthesis). It is an endogenous substance synthesized, stored and released in-
hormone, near its site of synthesis). It is an endogenous substance synthesized, stored and released in-
(a) mast cells, which are abundant in the skin, GI and the respiratory tract,
(b) basophils in the blood and
(c) some neurons in the CNS.
(b) basophils in the blood and
(c) some neurons in the CNS.
Biosynthesis of Histamine
Histamine is synthesized in cytoplasmic granules of its principal storage cells (mast cells & basophil) from naturally
occurring amino acid S-histidine via catalysis of pyridoxal phosphate dependant histidine decarboxylase.
occurring amino acid S-histidine via catalysis of pyridoxal phosphate dependant histidine decarboxylase.
Storage and release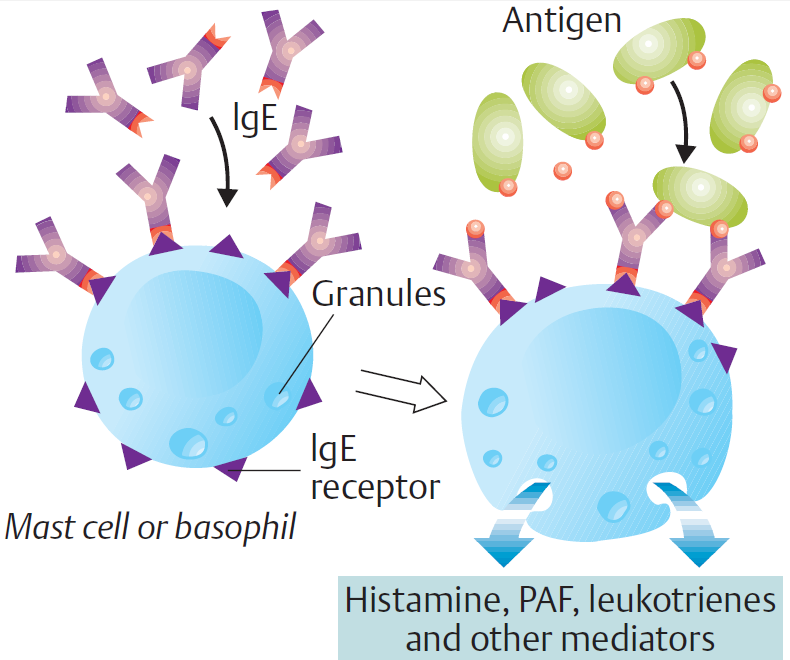
- Most histamine is synthesized & stored in mast cells & basophils.
- Histamine is also stored in selected neuronal tracts in the CNS.
- Protein-complexed histamine is stored in secretory granules & released by exocytosis in response to a wide variety
of immune & non-immune stimuli. - The stimuli for release of histamine from tissues may include destruction of cells as a result of cold, toxins from
organisms, venoms from insects and spiders, and trauma. - Allergies and anaphylaxis can also trigger significant release of histamine, where histamine release is initiated by
the interaction of an antigen-antibody (IgE) complex with the membrane of a histamine storage cell. - Exocytotic release of histamine follows the degranulation of the storage cell.
- Histamine is released from mast cells in the gastric mucosa by gastrin & acetylcholine.
Histamine Receptors
Four different histamine receptors have been characterized and are designated
H1 –H4 all of which are G protein-coupled receptors. These different receptors are expressed on different cell types and
work through different intracellular signalling mechanisms, which explain, at least at a simple level, the diverse effects of
histamine in different cells and tissues.
work through different intracellular signalling mechanisms, which explain, at least at a simple level, the diverse effects of
histamine in different cells and tissues.
Receptor
Type |
Major Tissue Locations
|
Major Biologic Effects
|
H1
|
Smooth muscle, Endothelial cells and Nerve endings.
|
Acute allergic responses, vasodilatation,
Contraction of most smooth muscle, except blood vessels. |
H2
|
Gastric parietal cells (gastric mucosa),
Cardiac muscle cells, Mast cells and Brain. |
Stimulation of gastric secretion.
|
H3
|
Central nervous system (Presynaptic autoreceptors
and heteroreceptors) |
Modulating neurotransmission
|
H4
|
Intestinal tissue, Spleen, Thymus &
Immune active cells such as- T cells, Neutrophils, Eosinophils. |
Regulating immune responses
|
The ‘triple response’. When injected intradermally, histamine causes a reddening of the skin (red spot), owing to dilation of small vessels, accompanied by a weal
(an edematous wheal) with a red irregular flare. This is the triple response described by Sir Thomas Lewis over 80 years ago and is explained by the foregoing effects.
(an edematous wheal) with a red irregular flare. This is the triple response described by Sir Thomas Lewis over 80 years ago and is explained by the foregoing effects.
H1 Receptor antagonists
The term antihistamine historically has referred to drugs that antagonize the actions of histamine at H1-receptors.
The H1-antagonists are now commonly subdivided into two broad groups - the first generation or classical antihistamines
and the second generation or “non-sedating” antihistamines – based primarily on their general pharmacological profiles.
The H1-antagonists are now commonly subdivided into two broad groups - the first generation or classical antihistamines
and the second generation or “non-sedating” antihistamines – based primarily on their general pharmacological profiles.
The first generation or classical antihistamines are related structurally and include a number of aminoalkyl ethers,
ethylenediamines, piperazines, propylamines, phenothiazines and dibenzocycloheptenes. In addition to H1-receptor
antagonism, these compounds display an array of other pharmacological activities which contribute toward
therapeutic applications and adverse reactions. More recently, a number of second generation or “non-sedating”
antihistamines have been developed and introduced. The second generation agents bear some structural resemblance
to the first generation agents, but have been modified to be more specific in action and limited in their distribution
profiles.
ethylenediamines, piperazines, propylamines, phenothiazines and dibenzocycloheptenes. In addition to H1-receptor
antagonism, these compounds display an array of other pharmacological activities which contribute toward
therapeutic applications and adverse reactions. More recently, a number of second generation or “non-sedating”
antihistamines have been developed and introduced. The second generation agents bear some structural resemblance
to the first generation agents, but have been modified to be more specific in action and limited in their distribution
profiles.
Histamine H1-Receptor Antagonists
(i) Aminoalkylethers : Examples-Diphenhydramine Hydrochloride ; Bromodiphenhydramine Hydrochloride ;
Dimenhydrinate ; Doxylamine Succinate ; Diphenylpyraline Hydrochloride.
Dimenhydrinate ; Doxylamine Succinate ; Diphenylpyraline Hydrochloride.
(ii) Ethylenediamines : Examples-Mepyramine Maleate ; Tripelennamine Hydrochloride, Thonzylamine Hydrochloride ;
Zolamine Hydrochloride.
Zolamine Hydrochloride.
(iii) Thiophene Derivatives: Examples-Methapyrilene Hydrochloride; Methaphenilene Hydrochloride, Thenyldiamine
Hydrochloride; Chlorothen Citrate.
Hydrochloride; Chlorothen Citrate.
(iv) Cyclic Basic Chain Analogues: Examples-
(a) Imidazoline Derivatives, e.g., Antazoline Hydrochloride ;
(b) Piperazine Derivatives, e.g., Cyclizine Hydrochloride ; Chlorcyclizine Hydrochloride ; Meclizine Hydrochloride ;
Buclizine Hydrochloride ;
(b) Piperazine Derivatives, e.g., Cyclizine Hydrochloride ; Chlorcyclizine Hydrochloride ; Meclizine Hydrochloride ;
Buclizine Hydrochloride ;
(c) Piperidine Derivativs, e.g., Thenalidine Tartrate.
(v) Phenothiazine Derivatives: Examples-Promethazine Hydrochoride ; Promethazine Teoclate ; Trimeprazine Tartrate ; Methdilazine Hydrochloride.
(vi) Second-generation Non-Sedating Antihistamines: Examples: Terfenadine ;
Astemizole ; Loratadine ; Acrivastine ;
(vii) Miscellaneous Agents: Examples-Phenindamine Tartrate; Triprolidine Hydrochloride; Chlorpheniramine Maleate;
Cyproheptadine Hydrochloride.
Cyproheptadine Hydrochloride.
SAR of H1 Receptor antagonists
General structure of first-generation antihistamines
- The diaryl substitution pattern is present in both the first and second generation antihistamines and is essential for
significant H1-receptor affinity. Most
H1-antagonists contain substituents in one of the aryl rings (usually benzene), and these influence antihistamine
potency, as well as bio disposition.
- In many of the first generation antihistamines the terminal nitrogen atom is a simple dimethyl amino moiety. However,
the amine may also be part of a heterocyclic structure, as illustrated by the piperazine, some propylamines
(pyrrolidines and piperdines), some phenothiazines, the dibenzocycloheptenes and the second generation
antihistamines. In all cases the amino moiety is basic with pKas ranging from 8.5 to 10 and thus presumed to be
protonated when bound on the receptor.
- X is a connecting atom of O, C or N. The X connecting moiety of typical
H1-antagonists may be a saturated carbon-oxygen moiety or simply a carbon or nitrogen atom. This group along
with the carbon chain appears to serve primarily as a spacer group for the key pharmacophoric moieties.
Many of the anthistamines containing a carbon atom in the connecting moiety are chiral, and exhibit stereoselective
receptor binding. For example, in the pheniramine series and carbinoxamine, this atom is chiral and in vitro analysis
indicates that those enantiomers with the S-configuration have higher H1-receptor affinity.
receptor binding. For example, in the pheniramine series and carbinoxamine, this atom is chiral and in vitro analysis
indicates that those enantiomers with the S-configuration have higher H1-receptor affinity.
- The (CH2)n group and connecting atom results in a distance between the central point of the diaryl ring system and
the terminal nitrogen atom in the extended conformation of the antihistamines ranging from 5 to 6 angstroms
(a "spacer" group). In some series branching of the carbon chain results in a reduction of antihistaminic activity.
However, there are exceptions as evidence by promethazine which has a greater activity than its
non-branched counterpart. - When the carbon adjacent to the terminal nitrogen atom is branched, the possibility of asymmetry exists. However,
stereoselective H1-receptor antagonism typically is not observed when chirality exists at this site. Also, in those
compounds which possess an asymmetrically substituted unsaturated carbon chain (pyrrobutamine and triprolidine)
one geometric isomer typically displays higher receptor affinity than the other.
Generally, the first and second generation anthistamines are substantially more lipophilic than the endogenous agonist
histamine (or the H2-antagonists).
This lipophilicity difference results primarily from the presence of the two aryl rings, and the substituted amino moieties,
and thus may simply reflect the different structural requirements for antagonist versus agonist action at H1-receptors.
histamine (or the H2-antagonists).
This lipophilicity difference results primarily from the presence of the two aryl rings, and the substituted amino moieties,
and thus may simply reflect the different structural requirements for antagonist versus agonist action at H1-receptors.
First generation H1 receptor blocker
| |
Ethers or Ethanolamine derivative
|
Ethylenediamine derivative
|
Piperazine derivative
|
Alkylamine derivative
|
Phenothiazine derivative
|
Piperidine derivatives(2nd Gen.)
|
Differences between first generation & second generation antihistamines
Features
|
First generation
H1 receptor blocker |
Second generation
H1 receptor blocker |
Daily Doses
|
Usually three to four daily doses
|
Usually once or twice a day
|
Blood-brain barrier
|
Cross the BBB
|
Don’t cross the BBB
|
Side effects
|
Potentially occurs
|
Do not cause relevant side effects
|
Common side effects
|
sedation/hyperactivity/insomnia/ convulsions
|
sedation/fatigue/hyperactivity/ convulsions
|
Toxicity
|
Case reports regularly published
|
No reports of serious toxicity
|
Lethal dose
|
Lethal dose identified for infants/young children
|
Do not cause fatality in overdose
|
H2 receptor blockers
Physiological regulation of acid secretion by the parietal cell / Physiology of acid secretion:
Gastric acid is secreted from parietal cells located mainly in the upper portion of the stomach and is stimulated
by three endogenous substances: gastrin, acetylcholine, and histamine. The parietal cell contains receptors for
gastrin, acetylcholine (muscarinic, M3), and histamine (H2). When acetylcholine or gastrin binds to the parietal cell
receptors, they cause an increase in cytosolic calcium, which in turn stimulates protein kinases that stimulate acid
secretion from an H+/K+ ATPase (the proton pump) on the canalicular surface.
by three endogenous substances: gastrin, acetylcholine, and histamine. The parietal cell contains receptors for
gastrin, acetylcholine (muscarinic, M3), and histamine (H2). When acetylcholine or gastrin binds to the parietal cell
receptors, they cause an increase in cytosolic calcium, which in turn stimulates protein kinases that stimulate acid
secretion from an H+/K+ ATPase (the proton pump) on the canalicular surface.
In close proximity to the parietal cells are gut endocrine cells called enterochromaffin-like (ECL) cells. ECL cells have
receptors for gastrin and acetylcholine. It is thought that gastrin and acetylcholine act on ECL cells to
release histamine. Histamine then binds to the H2 receptor on the parietal cell, resulting in activation of adenylyl cyclase,
which increases intracellular cyclic adenosine monophosphate (cAMP), cAMP activates protein kinases that stimulate
acid secretion by the H+/K+ ATPase.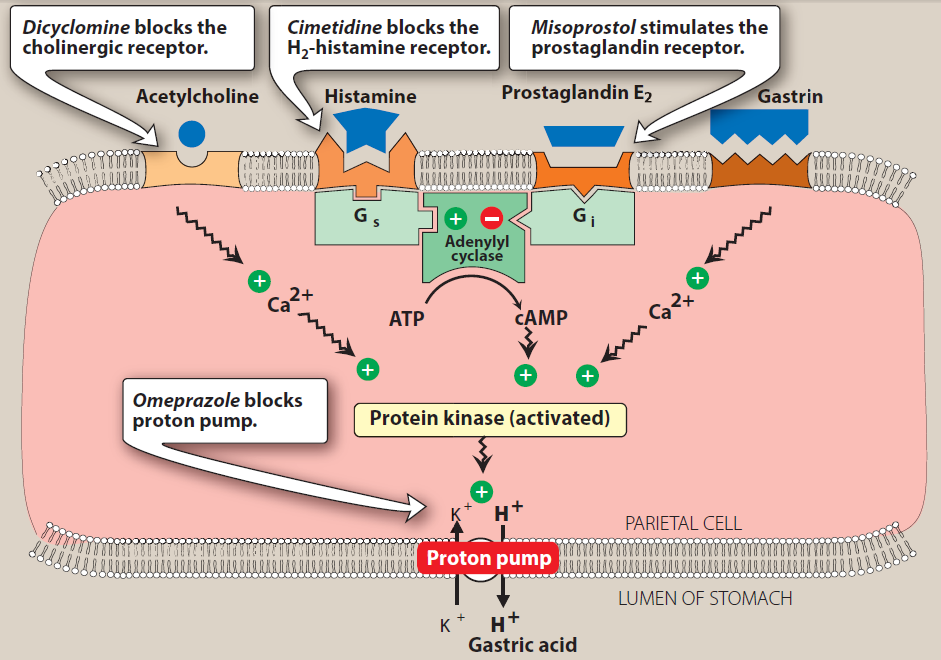
receptors for gastrin and acetylcholine. It is thought that gastrin and acetylcholine act on ECL cells to
release histamine. Histamine then binds to the H2 receptor on the parietal cell, resulting in activation of adenylyl cyclase,
which increases intracellular cyclic adenosine monophosphate (cAMP), cAMP activates protein kinases that stimulate
acid secretion by the H+/K+ ATPase.
Figure: Schematic diagram of one model of the physiologic control of hydrogen Ion secretion by the gastric parietal cell. EC cell, enterochromaffin-like cell;G
(CCK-B>, gastrih-chdlecystokinin-B receptor; H, histamine; H2. histamine Ha receptor; M1, M3, muscarinic receptors; ST2 somatostatin receptor; ATPase,
Kf/Ht ATPase proton pump.Some investigators place histamine receptors—and possibly cholinoceptors—on nearby tissue cells rather than on the parietal cell
Itself. (Modified and redrawn from Sachs 6,Prinz C:Gastric enterochromaffin-like cells and the regulation of acid secret.on.News Physiol Set 1996eU:S7,and
other sources)
(CCK-B>, gastrih-chdlecystokinin-B receptor; H, histamine; H2. histamine Ha receptor; M1, M3, muscarinic receptors; ST2 somatostatin receptor; ATPase,
Kf/Ht ATPase proton pump.Some investigators place histamine receptors—and possibly cholinoceptors—on nearby tissue cells rather than on the parietal cell
Itself. (Modified and redrawn from Sachs 6,Prinz C:Gastric enterochromaffin-like cells and the regulation of acid secret.on.News Physiol Set 1996eU:S7,and
other sources)
H2 receptor antagonists
Chemistry:
The H2 receptor antagonists in clinical use are histamine congeners that contain a bulky side chain in place of the
ethylamine moiety. Early representatives of the group, such as burimamide and cimetidine (the first compound released
for general use) retain the imidazole ring of histamine. This ring is replaced in more recently developed compounds by
a furan (ranitidine) or a thiazole (famotidine, nizatidine). Structures of histamine and some H2 antagonists are given
below:
ethylamine moiety. Early representatives of the group, such as burimamide and cimetidine (the first compound released
for general use) retain the imidazole ring of histamine. This ring is replaced in more recently developed compounds by
a furan (ranitidine) or a thiazole (famotidine, nizatidine). Structures of histamine and some H2 antagonists are given
below:
Mechanism of action of H2 receptor antagonists
The H2 receptor antagonists exhibit competitive inhibition at the parietal cell
H2 receptor, and suppress basal (fasting), nocturnal and meal stimulated acid secretion in a linear dose-dependent
manner. They are highly selective and do not affect H1 or H3 receptors
H2 receptor, and suppress basal (fasting), nocturnal and meal stimulated acid secretion in a linear dose-dependent
manner. They are highly selective and do not affect H1 or H3 receptors
H2 antagonists reduce acid secretion stimulated by histamine as well as by gastrin and cholinomimetic agents through
two mechanisms:
two mechanisms:
First, histamine released from ECL cells by gastrin or vagal stimulation is blocked from binding to the parietal cell H2
receptor.
receptor.
Second, direct stimulation of the parietal cell by gastrin or acetylcholine results in diminished acid secretion in the
presence of H2 receptor blockade. It appears that reduced parietal cell cAMP levels attenuate the intracellular
activation of protein kinases by gastrin or acetylcholine.
presence of H2 receptor blockade. It appears that reduced parietal cell cAMP levels attenuate the intracellular
activation of protein kinases by gastrin or acetylcholine.
References:
- Katzung, Bertram G., Susan B. Masters, and Anthony J. Trevor. Basic & Clinical Pharmacology. 12th ed. New York:
McGraw-Hill Medical, 2012. - Goodman, Louis S, Alfred Gilman, and Laurence L Brunton.
Goodman & Gilman's Manual of Pharmacology and Therapeutics.
New York: McGraw-Hill Medical, 2008.
- Remington, Joseph P. Remington, the Science and Practice of Pharmacy. Easton, Pa: Mack Pub. Co, 1995.
- Richard Finkel, Michelle A. Clark, Luigi X. Lippincott's Illustrated Reviews: Pharmacology. 6th ed. Baltimore, MD; New Delhi:
Wolters Kluwer Health/Lippincott Williams & Wilkins, 2012.
- H P Rang, M M Dale, J M Ritter, R J Flower, G Henderson RANG AND DALE’S Pharmacology. 7th ed. Elsevier Inc.
2007.
Get the PDF and PowerPoint slides
Antazoline hydrochloride is an antagonist of histamine H1 receptors, with anticholinergic properties. It is used to relieve nasal congestion and in eye drops. Antazoline Hydrochloride Ph. Eur.
ReplyDeleteVery Informative content on Diphenhydramine Thank you for the article!
ReplyDelete