SOLUTION
Definitions
A solution may be defined as a homogeneous mixture of two or more components that form a single phase.
The component that determines the phase of the solution is termed the solvent and usually constitutes the largest proportion of the system. The other components dispersed as molecules or ions
throughout the solvent are termed solutes.
throughout the solvent are termed solutes.
The transfer of molecules or ions from a solid state into solution is known as dissolution. The extent to which the dissolution proceeds under a given set of experimental conditions is referred to as
the solubility of the solute in the solvent.
the solubility of the solute in the solvent.
Solute molecules
Solvent molecules
Fig. Molecular model of a solution
Physical Properties of Solutions
1. Colligative properties
- The properties which depend on the number of particles in the solution (i.e. relative proportions of the molecules of the solute and solvent) and not on the size or chemical nature of the
particles are called Colligative properties
particles are called Colligative properties
Boiling Point elevation, Freezing Point depression, Vapor Pressure lowering, Osmotic Pressure
- The values are same for equal concentrations of different nonelectrolytes in solution.
- Related to each other.
2. Additive properties
- The properties of substance which depends upon the total contribution of the atoms or functional groups within the molecules are called additive properties. e.g Mass, Molecular weight (sum
of masses of each atom in the molecule).
of masses of each atom in the molecule).
3. Constitutive properties
- The properties which mainly depend on the structural arrangement, number and kinds of atoms in molecules are called constitutive properties.
- e.g. Optical rotation – depends on the chirality of the molecule which is determined by the atomic bonding order; surface tension, viscosity etc.
Types of Solution
i.) Based on the amount of solute -
a) Saturated Solution
Saturated solution is a solution to which no more solute can be added at a particular temperature.
As temperature affects the solubility of a substance, a saturated solution at lower temperature may become a dilute solution at higher temperature.
As temperature affects the solubility of a substance, a saturated solution at lower temperature may become a dilute solution at higher temperature.
b) Unsaturated Solution
It is a solution to which more solute can be added to dissolve. They are also otherwise called dilute solutions. An unsaturated solution contains less solute than the solvent has the capacity to
dissolve at a specific temperature.
dissolve at a specific temperature.
c) Supersaturated solution
A solution that contains a higher than saturation concentration of solute; slight disturbance or seeding causes crystallization of excess solute.
The solubility of the salt will increase with increase in temperature. Hence we can add more solute to the saturated solution at an elevated temperature (nearly at its boiling point). A solution
where the solute cannot be added further even at elevated temperature is called a super saturated solution. It is highly unstable and the solute will crystallize when the temperature is reduced. A
supersaturated contains more solute than is present in a saturated solution at a specific temperature.
where the solute cannot be added further even at elevated temperature is called a super saturated solution. It is highly unstable and the solute will crystallize when the temperature is reduced. A
supersaturated contains more solute than is present in a saturated solution at a specific temperature.
ii) Based on the physical state of the solutes and solvents
Sl.
|
Solute
|
Solvent
|
Example
|
01.
|
Gas
|
Gas
|
Air
|
02.
|
Liquid
|
Gas
|
Water in oxygen
|
03.
|
Solid
|
Gas
|
Iodine vapour in air
|
04.
|
Gas
|
Liquid
|
Carbonated water
|
05.
|
Liquid
|
Liquid
|
Alcohol in water
|
06.
|
Solid
|
Liquid
|
Aqueous NaCl solution
|
07.
|
Gas
|
Solid
|
Hydrogen in palladium
|
08.
|
Liquid
|
Solid
|
Hg in Ag, Mineral oil in paraffin
|
09.
|
Solid
|
Solid
|
Gold-silver mixture
|
Ideal and real solutions
- Ideal solution is defined as a solution in which there is no change in the properties of the components other than dilution when they are mixed to form the solution
- Molecules exhibit complete freedom of motion and randomness of distribution in the solution.
- Ideality in solutions means complete uniformity of attractive forces
Concentration of a Solution
The concentration of a solution is defined as: the amount of solute present in a given amount of solution.
Concentration is a general measurement unit stating the amount of solute (solu.) present in a known amount of solution (soln.)
Concentration=amount of solute (solu.)amount of solution (soln.)
A solution containing a relatively low concentration of solute is called Dilute solution. A solution of high concentration is called Concentrated solution.
Concentration Expression
Expression Symbol Definitions
Molarity M Number of moles (gm molecular weight) of solute in 1 L of solution.
Normality N Gram equivalent weights of solute in 1 L of solution
Molality m Moles of solute in 1000 g solvent
Mole Fraction X Ratio of moles of one constituent of a solution to the total moles of all constituents (solute & solvent)
Mole percent -- Moles of one constituent in 100 moles of the solution. It is obtained by multiplying X by 100
Percent by weight %w/w Gram of solute in 100 gm solution
Percent by volume %v/v ml of solute in 100 ml of solution
Percent by weight-in-volume %w/v Gram of solute in 100 ml solution
Milligram percent --- mg of solute in 100 ml of solution
Molarity (M)
Molarity is the number of moles of solute dissolved per liter of solution.
Problems
•What is the molarity of a solution prepared by dissolving 75.5 g of pure KOH in 540 ml of solution?
•Calculate the amount of HCl present in 150 ml of a 0.5 M solution?
Normality (N)
Number of gram equivalent (eq.) weights of solute in 1L of solution.
N=eq. of solu. (eq)vol. of soln. (L)
Relationship between molarity and normality for the same solute in the same solution:
M=molVol. (L)=(Wt.M.M.)Vol. (L), N=eq.Vol. (L)= (Wt.Eq. M.)Vol. (L)
So
Eq. M.=M.M.n ⇒ N=(Wt.M.M.n)Vol. (L)=n x (Wt.M.M.)Vol. (L)
Thus: N=n M n ≥ ≥1 therefore N ≥ M
Equivalent mass (Eq. M.): equivalent mass of a substance can be calculated by dividing its molar mass per the number of active units in one molecule of this substance (n) thus:
Eq. M.=M.M.no. of active unit in one molecule of solu. (n)
The active unit in the acid-base reactions is the number of hydrogen ions liberated by a single molecule of an acid or reacted with a single molecule of a base.
Acid: one definition of acid is any substance that ionizes in water to form H+, includes monoprotic and polyprotic. Monoprotic acid like hydrochloric acid (HCl) which includes only one proton
(hydrogen ion), and polyprotic acid like sulfuric acid (H2SO4) that contains two protons (hydrogen ion).
(hydrogen ion), and polyprotic acid like sulfuric acid (H2SO4) that contains two protons (hydrogen ion).
The active unit in the oxidation-reduction (redox) reactions is the number of electrons (e) transferred from one reactant to another during the reaction.
A 3M H2SO4 solution is the same as a 6N H2SO4 solution.
For a basic solution, n is the number of OH- ions provided by a formula unit of base.
A 1M Ca(OH)2 solution is the same as a 2N Ca(OH)2 solution
For a basic solution, n is the number of OH- ions provided by a formula unit of base.
A 1M Ca(OH)2 solution is the same as a 2N Ca(OH)2 solution
Problems for using Molarity and Normality
1. Volume of solution change with temperature. So it should not be used when one wishes to study the properties of solutions at various temperatures.
2. Difficult to study the properties of solutions such as vapor pressure and osmotic pressure which are related to the concentration of the solvent.
Problem
•Calculate the amount required to produce 250ml, 1N HCl solution. The purity of HCl 37.0 % & the density of the reagent is 1.19 g/mL.?
•Calculate the amount required to produce 250ml, 1N HCl solution. The purity of HCl 37.0 % & the density of the reagent is 1.19 g/mL.?
•Find the Normality of 0.67 g. KOH in 120 mL of water?
Molality (m)
Number of moles of solute in 1000 g solvent. It does not change with temperature.
m=mole of solute (mol)mass of solvent (kg)
m=mole of solute (mol)mass of solvent (kg)
A solution obtained by dissolving one mole of the solute in 1000 g of solvent is called one molal or 1m solution.
Problem
•What is the molality of a solution prepared by dissolving 50.0 g of HCl in 225 g of water?
Mole Fraction(X)
Mole fraction is the ratio of the number of moles of one constituent (solute) in solution to the total number of moles of solute and solvent.
Where XA and XB represent mole fractions of each substance
Mole Percent
Moles of one constituent in 100 moles of the solution. It is obtained by multiplying ‘X’ by 100.
Percent by weight in weight (%w/w)
It is the weight of the solute as a per cent of the total weight of the solution. That is,
For example, if a solution of HCl contains 36 per cent HCl by weight, it has 36 g of HCl for 100g of solution.
Problem
•What is the per cent by weight of NaCl if 2.75 g of NaCl is dissolved in 9.85 g of water?
Percent by volume in volume (%v/v)
ml of solute in 100 ml of solution
%vv=Volume of soluteVolume of solution ×100
Problem
•Make 1000ml of a 5% by volume solution of ethylene glycol in water.
•Make 1000ml of a 5% by volume solution of ethylene glycol in water.
✍Procedure
First, express the percent of solute as a decimal: 5% = 0.05
Multiply this decimal by the total volume: 0.05 x 1000ml = 50ml (ethylene glycol needed).
Subtract the volume of solute (ethylene glycol) from the total solution volume:
1000ml (total solution volume) - 50ml (ethylene glycol volume) = 950ml (water needed)
Dissolve 50ml ethylene glycol in a little less than 950ml of water. Now bring final volume of solution up to 1000ml with the addition of more water. (This eliminates any error because the final
volume of the solution may not equal the calculated sum of the individual components).
volume of the solution may not equal the calculated sum of the individual components).
So, 50ml ethylene glycol / 1000ml solution x100 = 5% (v/v) ethylene glycol solution.
Percent by weight-in-volume (%w/v)
Gram of solute in 100 ml solution
%wv=Weight of soluteVolume of solution ×100
•15mL of an aqueous solution of sucrose contains 750mg sucrose. What is the weight/volume percentage concentration of this solution in g/100mL?
Dilution: addition of solvent to decrease the concentration of solute. The solution volume changes, but the amount of solute is constant.
moles of solute (mol) = molarity (M) x volume (V)
M1V1 = M2V2
Initial values final values
Problem
•Prepare 250 mL of 0.100 M NaCl solution from a 2.00 M NaCl solution.
M1 = molarity of starting solution (in this case 2.00M NaCl)
V1 = volume of starting solution required (always unknown)
M2 = molarity of final solution after dilution (in this case 0.100M NaCl)
V2 = volume of final solution, after dilution (in this case 250ml)
V1 = M2 V2 / M1
V1 = (0.100M) x (250 ml) / (2.00M) = 12.5ml
To make molar NaCl solutions of other concentrations dilute the mass of salt to 1000ml of solution as follows:
0.1M NaCl solution requires 0.1 x 58.44 g of NaCl = 5.844g
0.5M NaCl solution requires 0.5 x 58.44 g of NaCl = 29.22g
2M NaCl solution requires 2.0 x 58.44 g of NaCl = 116.88g
Solutions of Solids in Liquid
Will sugar dissolve in water? Will petroleum dissolve in water?
Like Dissolves Like
Polar molecules are soluble in polar solvents C2H5OH in H2O
Non-polar molecules are soluble in non-polar solvents e.g. CCl4 in C6H6
Polar compounds, like table sugar (C12H22O11), are soluble in polar solvents and insoluble in nonpolar solvents.
Nonpolar compounds, like naphthalene (C10H8), are soluble in nonpolar solvents and insoluble in polar solvents.
This it the like dissolves like rule.
Ionic compounds, like sodium chloride, are soluble in polar solvents and insoluble in nonpolar solvents.
An ionic solid dissolves in water because the number of water molecules around the surface is greater than the number of other ions of the solid. The attraction between polar water molecules and
a charged ion enables the water molecules to pull ions away from the crystal, a process called dissolving.
An ionic solid dissolves in water because the number of water molecules around the surface is greater than the number of other ions of the solid. The attraction between polar water molecules and
a charged ion enables the water molecules to pull ions away from the crystal, a process called dissolving.
The mechanism of solution of sodium crystal in water could be explained as follows. The polar water molecules try to pull out the Na+ and Cl– ions from the crystal by hydration. This becomes
possible since the forces operating between the ion (Na+ or Cl–) and water molecules are strong enough to overcome the force binding the ion in the crystal. The ions detached from the crystal are
now surrounded by cluster of water molecules. The layer of water molecules enveloping the ions effectively shields them and prevents them from coming in contact with each other. Thus they
hardly aggregate into a crystal and remain in solution.
possible since the forces operating between the ion (Na+ or Cl–) and water molecules are strong enough to overcome the force binding the ion in the crystal. The ions detached from the crystal are
now surrounded by cluster of water molecules. The layer of water molecules enveloping the ions effectively shields them and prevents them from coming in contact with each other. Thus they
hardly aggregate into a crystal and remain in solution.
Miscible & Immiscible
- Two liquids that completely dissolve in each other are miscible liquids.
- Two liquids that are not miscible in each other are immiscible liquids.
- Polar water and nonpolar oil are immiscible liquids and do not mix to form a solution.
Partition Coefficient
A partition-coefficient (P) is the ratio of concentrations of a compound in a mixture of two immiscible phases at equilibrium.Partition coefficient is generally defined as the fraction of drug in an
oil phase to that of an adjacent aqueous phase.
oil phase to that of an adjacent aqueous phase.
FORMULA
Partition coefficient(p)=Concentration of solute in oil phaseConcentration of solute in aqueous phase
Partition coefficient(p)=Concentration of solute in oil phaseConcentration of solute in aqueous phase
Accordingly compounds with relatively high partition coefficient are predominantly lipid soluble and consequently have very low aqueous solubility.
Compounds with very low partition coefficients will have difficulty in penetrating membranes resulting poor bioavailability.
Factors Affecting Solubility of a Solute
- Temperature
Generally in many cases solubility increases with the rise in temperature and decreases with the fall of temperature but it is not necessary in all cases. However we must follow two behaviours:
In endothermic process solubility increases with the increase in temperature and vice versa.
For example: solubility of potassium nitrate increases with the increase in temperature.
For example: solubility of potassium nitrate increases with the increase in temperature.
In exothermic process solubility decrease with the increase in temperature.
For example: solubility of calcium oxide decreases with the increase in temperature.
For example: solubility of calcium oxide decreases with the increase in temperature.
- Effect of pressure
The effect of pressure is observed only in the case of gases.
An increase in pressure increases of solubility of a gas in a liquid.
For example carbon di oxide is filled in cold drink bottles (such as coca cola, Pepsi 7up etc.) under pressure.
- Chemical natures of the solute and solvent
A polar solute will dissolve in a polar solvent but not in a nonpolar solvent. The adage "like dissolves like" is very useful.
Example: Alcohol (polar substance) dissolves in water (polar substance). Water (polar substance) does not dissolve in oil (nonpolar substance)
- Stirring
With liquid and solid solute, stirring brings fresh portions of the solvent in contact with the solute, thereby increasing the rate of dissolution.
- Amount of solute already dissolved
When there is little solute already in solution, dissolving takes place relatively rapidly. As the solution approaches the point where no solute can be dissolved, dissolving takes place more slowly.
- Molecular size
The larger the molecules of the solute are, the larger is their molecular weight and their size. If the pressure and temperature are the same than out of two solutes of the same polarity, the one
with smaller particles is usually more soluble.
with smaller particles is usually more soluble.
Nernst’s Distribution Law
Nernst gave a generalization governing the distribution of a solute between two non-miscible solvents. This is called Nernst’s Distribution law or Nernst’s Partition law or simply Distribution law or
Partition law.
Partition law.
Statement of Nernst’s distribution law: If a solute X distributes itself between two immiscible solvents A and B at constant temperature and X is in the same molecular condition in both solvents,
Thus, Concentration of X in A Concentration of X in B =KD
Or,  =KD
=KD
Where, C1 & C2 denotes the concentration of the solute in solvent A and B respectively.
Explanation: If we take two immiscible solvents A and B in a beaker, they form separate layers. When a solute X which is soluble in both solvents is added, it gets distributed or partitioned
between them. Molecules of X pass from solvent A to B and from solvent B to A. Finally a dynamic equilibrium is set up. At equilibrium, the rate, at which molecules of X pass from one solvent
to the other is balanced.
between them. Molecules of X pass from solvent A to B and from solvent B to A. Finally a dynamic equilibrium is set up. At equilibrium, the rate, at which molecules of X pass from one solvent
to the other is balanced.
Figure: Distribution of solute X between solvent A and B.
Now according to Nernst’s distribution law the distribution of solute x between solvent A and B
Concentration of X in A Concentration of X in B =a constant
Solubilities and distribution law
When a solute is shaken with two non-miscible solvents, at equilibrium both the solvents are saturated with the solute. Since the solubility also represents concentration, we can write the
distribution law as
distribution law as
Where S1 and S2 are the solubilities of the solute in the two solvents.
Derivation of distribution law (Distribution law is an equilibrium law.)
Let us take two immiscible solvents A and B in a beaker. Now add a solute X which is soluble in both of the solvents, thus it gets distributed or partitioned between them. Molecules of X pass
from solvent A to B and from solvent B to A. Finally a dynamic equilibrium is set up.
from solvent A to B and from solvent B to A. Finally a dynamic equilibrium is set up.
Fig. At equilibrium, the number of molecules of X passing from solvent A into B is proportional to its concentration in A and vice versa. Also, the rate of migration of solute molecules from A to
B and B to A is equal (Illustration).
B and B to A is equal (Illustration).
When the distribution of the solute X has reached dynamic equilibrium, the rate (R1) at which molecules of X pass from solvent A to B is proportional to its concentration (C1) in A.
That is, R1 ∝ C1
or R1= k1✕C1 where k1 is a constant
The rate (R2) at which molecules of X pass from solvent B to A is proportional to its concentration (C2) in B.
R2 ∝ C2
or R2 = k2✕C2 where k2 is a constant
Also, at equilibrium, the rate of migration of solute from one solvent to the other is equal.
Thus we have, R1= R2
or, k1✕C1 = k2✕C2
or, 
or, 
If temperature is fixed the distribution coefficient KD is also constant, since k1 and k2 are constants at the same temperature.
This is the Nernst’s Distribution law equation.
This is the Nernst’s Distribution law equation.
Limitations of distribution law
The conditions to be satisfied for the application of the Nernst’s Distribution law are :
1. Constant temperature. The temperature is kept constant throughout the experiment.
2. Same molecular state. The molecular state of the solute has to be the same in the two solvents. The law does not hold if there is association or dissociation of the solute in one of the solvents
.
.
3. Equilibrium concentrations. The concentrations of the solute are noted after the equilibrium has been established.
4. Dilute solutions. Suitable if solute concentration is low in two solvents. The law does not hold when the concentrations are high.
5. Non-miscibility of solvents. The two solvents should be non-miscible or only slightly soluble in each other. The extent of mutual solubility of the solvents remains unaltered by the addition of
solute to them.
solute to them.
Henry’s Law – A Form of Distribution Law
The relationship between pressure and solubility of a gas in a particular solvent was investigated by William Henry (1803). He gave a generalisation which is known as Henry’s Law. It may be
stated as
stated as
Henry’s law states: at a constant temperature the solubility of a gas in a liquid is proportional to the pressure of the gas above it. Henry’s law may be mathematically expressed as
Mathematically, Henry’s Law may be expressed as
C ∝ P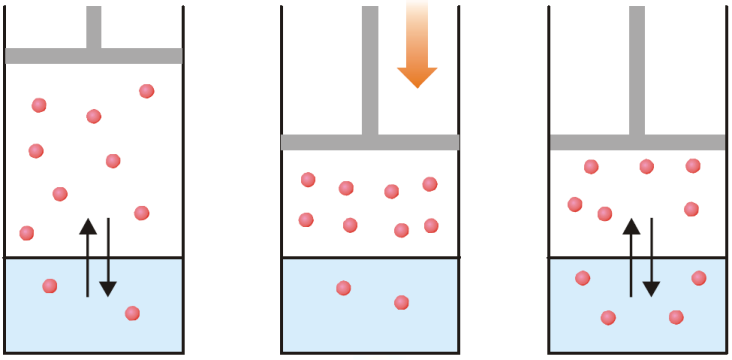
Or C = k P
Where P = pressure of the gas;
C = concentration of the gas in solution; and
k = proportionality constant known as Henry’s Law Constant. 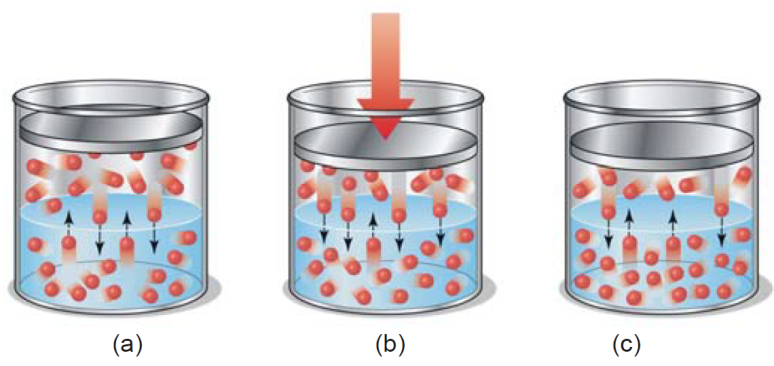
The value of k depends on the nature of the gas and solvent, and the units of P and C used.
(a) Equilibrium exists between the gas molecules and those in solution; (b) When pressure is applied, gas volume decreases and concentration in gas phase increases;
(c) More gas molecules pass into solution to re-establish equilibrium whereby the pressure above the gas is decreased.
Derivation
Henry’s law is, is a form of Distribution law. If a vessel containing a liquid and a gas is shaken, at equilibrium the gas can be regarded as distributed between the liquid (Phase A) and the space
above (Phase B).
above (Phase B).
Let C1 be the molar concentration of the gas in phase B
C be the concentration of the gas in phase A
Applying the Distribution law, 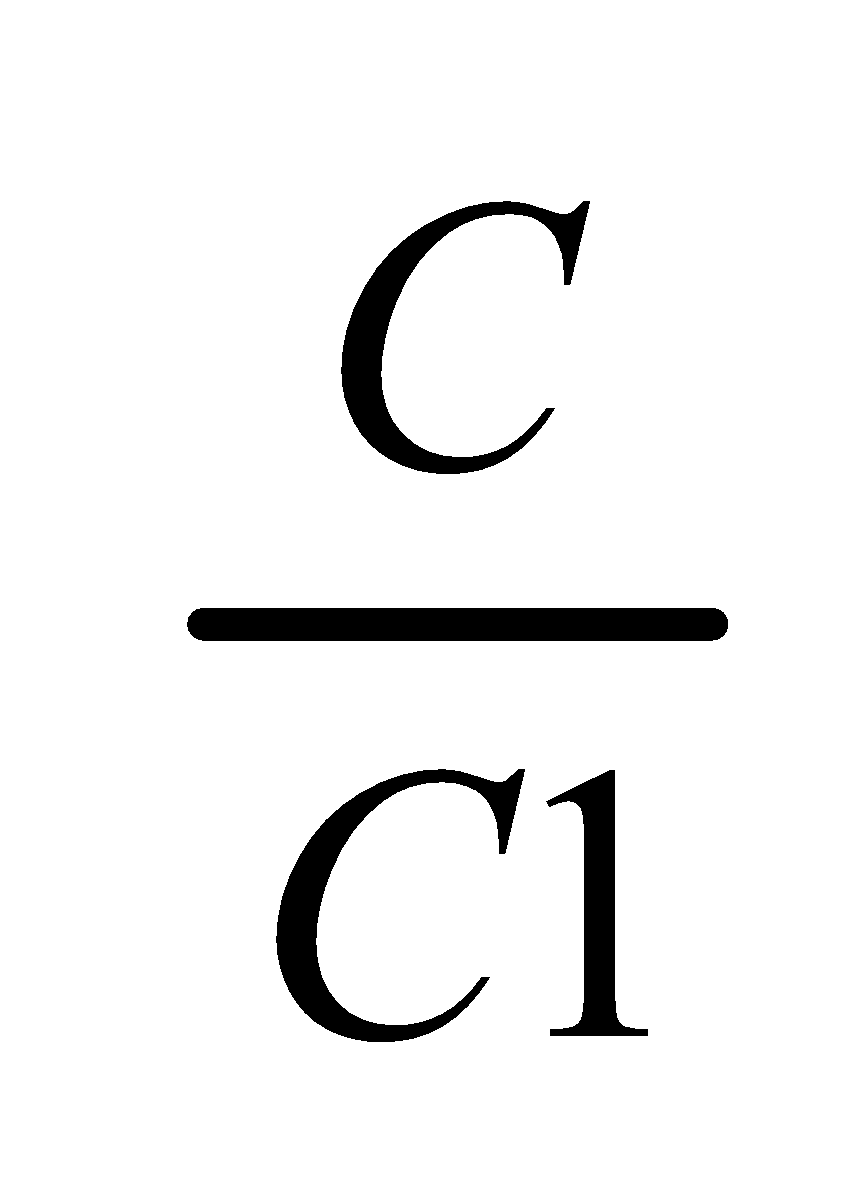 =KD (a constant)
=KD (a constant)
We know that molar concentration of gas is proportional to its pressure, P.
or C = K✕P
This is Henry’s Law equation.
Like distribution law, Henry’s law holds good for dilute solutions of gases which do not react with the solvent.
Limitations of Henry’s Law
It applies closely to gases with nearly ideal behaviour.
(1) At moderate temperature and pressure.
(2) If the solubility of the gas in the solvent is low.
(3) The gas does not react with the solvent to form a new species. Thus ammonia (or HCl) which react with water does not obey Henry’s Law
NH3 + H2O ⇌NH4+ + OH–
(4) The gas does not associate or dissociate on dissolving in the solvent.
(4) The gas does not associate or dissociate on dissolving in the solvent.
Use
The influence of partial pressure on solubility is utilized in making carbonated beverages like beer, champagne, and many soft drinks. So called ‘soda water’ is bottled under a carbon dioxide
pressure of about 4 atm. When the bottle is opened to the air, the partial pressure of CO2 above the solution is decreased (about 0.001 atm), and CO2 bubbles out.
pressure of about 4 atm. When the bottle is opened to the air, the partial pressure of CO2 above the solution is decreased (about 0.001 atm), and CO2 bubbles out.
(a ) CO gas at 4 atm in equilibrium with dissolved CO resulting in high solubility of CO ; (b ) In opened bottle pressure is released to 1 atm and hence equilibrium shifted upward, gas bubbles evolved
causing brisk effervescence; ( c) Partial pressure of CO in air being 0.001 atm, practically the whole of CO is removed from solution, leaving the soft drink flat as the equilibrium is established.
Applications of Distribution Law
There are numerous applications of distribution law in the laboratory as well as in industry. Here we will discuss some more important ones by way of recapitulation.
(1) Solvent Extraction
The removal of a substance(s) {solute} by a solvent of an organic substance from an aqueous solution is called extraction.
Classification
- Simple extraction: when the process of extraction is carried out with the total amount of the given solvent in a single operation, is referred to as simple extraction.
- Multiple extraction or multi-step extraction: To recover the maximum amount of the substance from aqueous solution, when the extraction is made in two or more successive operations
using small portions of the provided solvent, then it is called multiple extraction or multi-step extraction.
Simple extraction
The process of Simple extraction of organic substances from aqueous solutions is carried out by shaking the aqueous solution with a immiscible organic solvent, say ether (ethoxyethane), in a
separatory funnel.
separatory funnel.
The distribution ratio being in favour of ether, most of the organic substance passes into ether layer.
On standing, the aqueous and ether layers separate in the funnel. The lower aqueous layer is run out, leaving the ether layer behind. This is then transferred to a distillation flask. Ether is distilled
over while the organic substance is left as residue in the flask.
over while the organic substance is left as residue in the flask.
Fig. Extraction in a separatory funnel
If desired, the process may be repeated with aqueous layer left after the first extraction with a fresh quantity of the solvent.
The other common solvents used for extraction are hexane, benzene, chloroform, acetone, carbon disulphide, etc.
The greater the distribution ratio is in favour of the organic solvent, the greater will be the amount extracted in any one operation.
Multiple extractions
In Multiple extractions process the aqueous solution is first extracted with a portion of the solvent in a separatory funnel. The aqueous layer from which some substance has been removed is then
transferred to another funnel. This is shaken with another ( second) portion of the solvent. Similarly, the aqueous layer from the second extraction is treated with a third portion of solvent, and so
on.
transferred to another funnel. This is shaken with another ( second) portion of the solvent. Similarly, the aqueous layer from the second extraction is treated with a third portion of solvent, and so
on.
Superiority of multiple extraction over simple extraction
(** For better extraction, multiple extraction is more efficient than single extraction.)
(** For better extraction, multiple extraction is more efficient than single extraction.)
Generally it is more efficient to use a specified volume of solvent in small portions rather than in one whole.
Suppose we have 100 ml of an aqueous solution containing A grams of an organic substance.
We can extract the substance with ether (ethoxyethane), its distribution ratio being twice in favour of ether. Provided that 100 ml of ether which may be used in one lot or in two portions of 50
ml each.
We can extract the substance with ether (ethoxyethane), its distribution ratio being twice in favour of ether. Provided that 100 ml of ether which may be used in one lot or in two portions of 50
ml each.
- Using all the ether in one lot.
Let x grams be the weight of the substance extracted in the solvent layer. Then the amount of substance left in the water layer = (A – x) grams.
Therefore,
Concentration in ether layer = 
Concentration in water layer = 
According to the Distribution law 
Hence, x = 
Thus 66% of substance is extracted.
- Using two 50 ml portions of ether.
Let x1 grams of substance be extracted in the first operation with 50 ml ether.
Thus,
Concentration in ether layer= 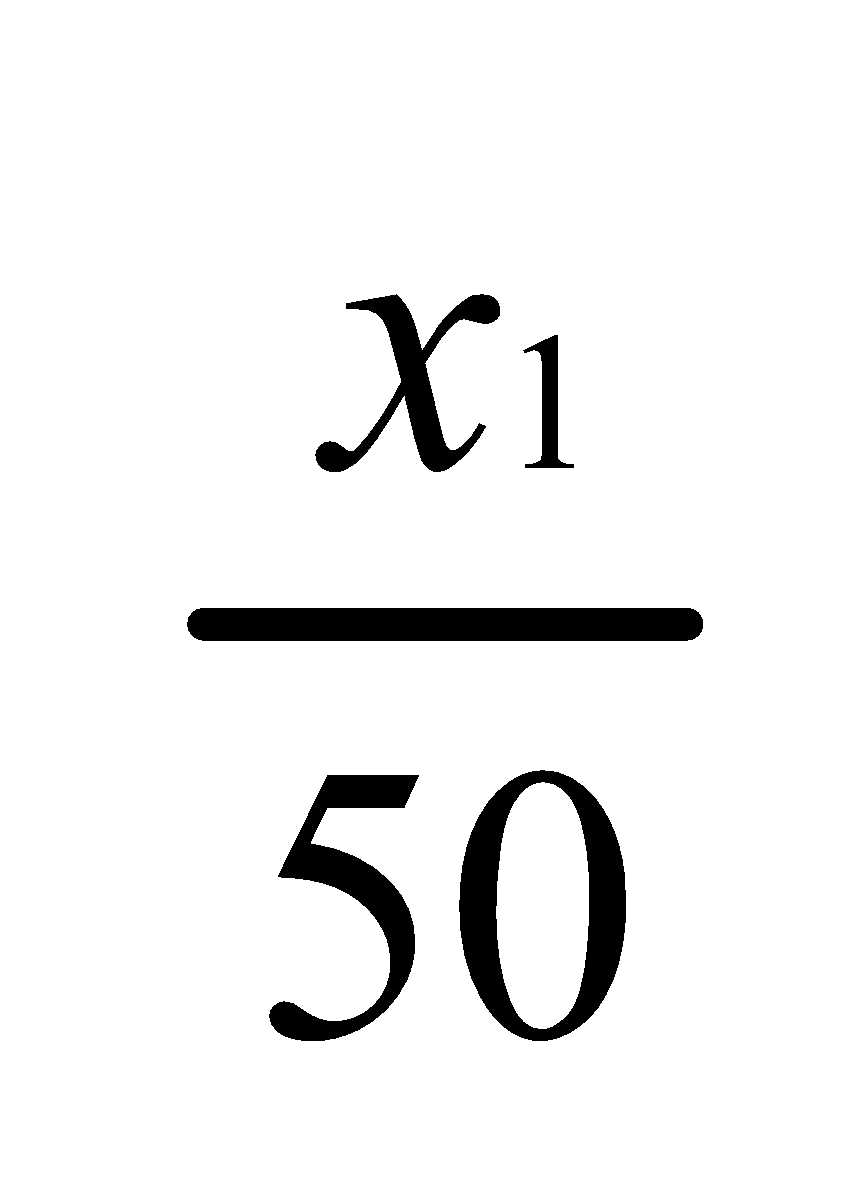
Concentration in water layer = 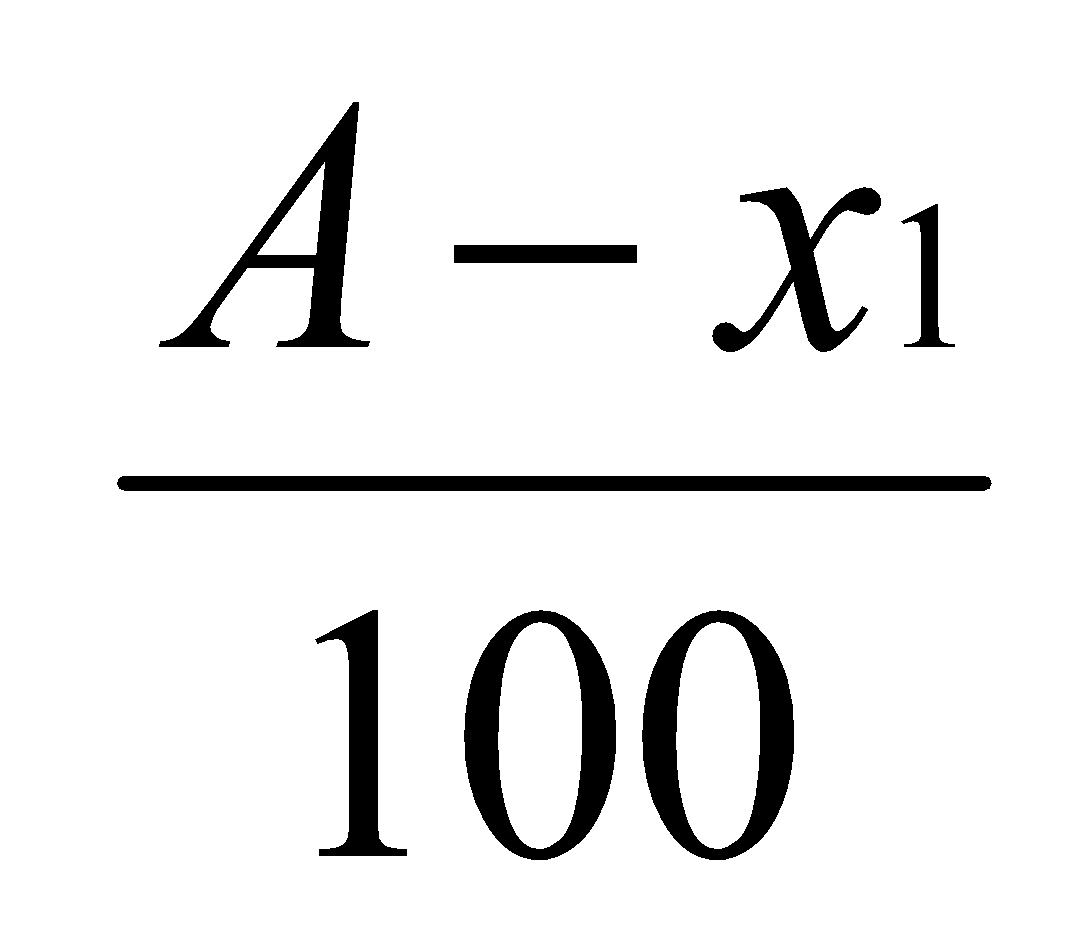
According to the Distribution law 
Thus,  Where K=2
Where K=2
Hence 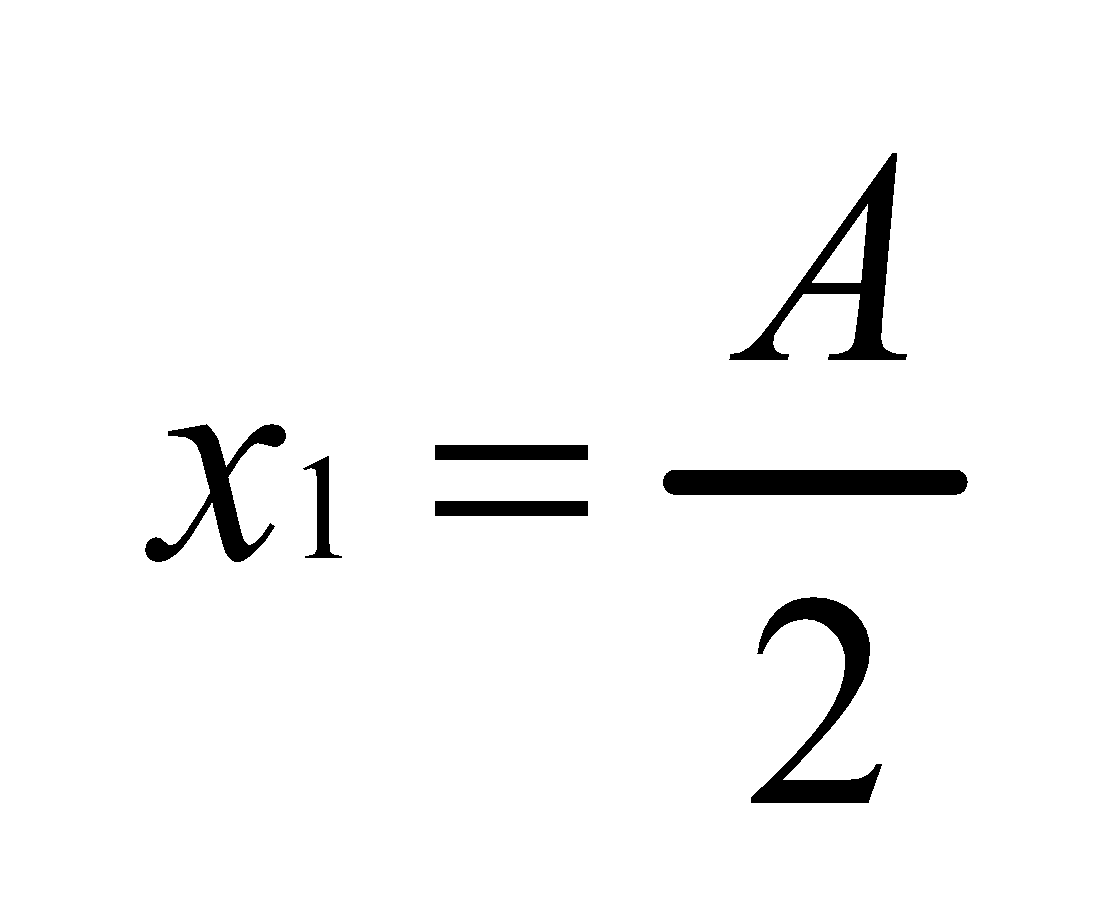
∴50% of substance is extracted.
The substance left in water layer is 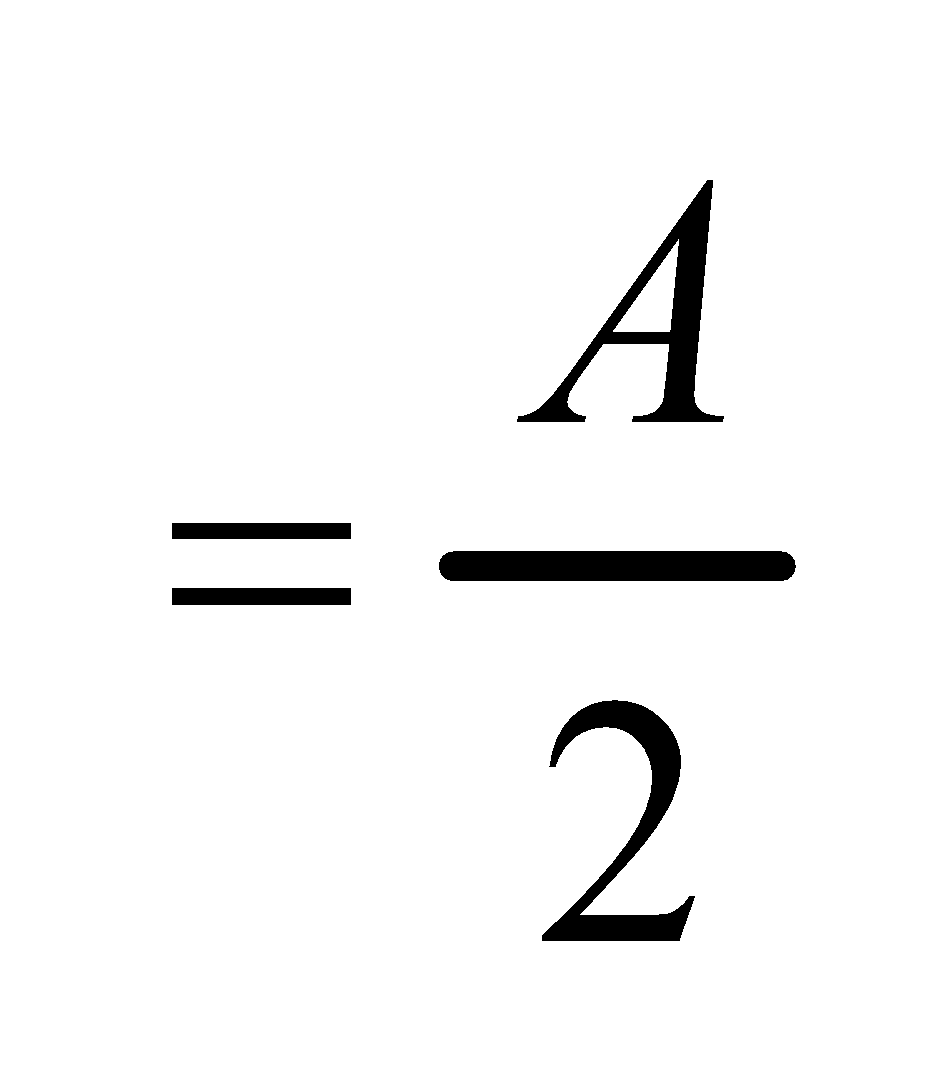
Let x2 grams be the substance removed from the water layer when it is extracted with another 50 ml portion of ether.
Hence,
Concentration in ether layer = 
Concentration in water layer = 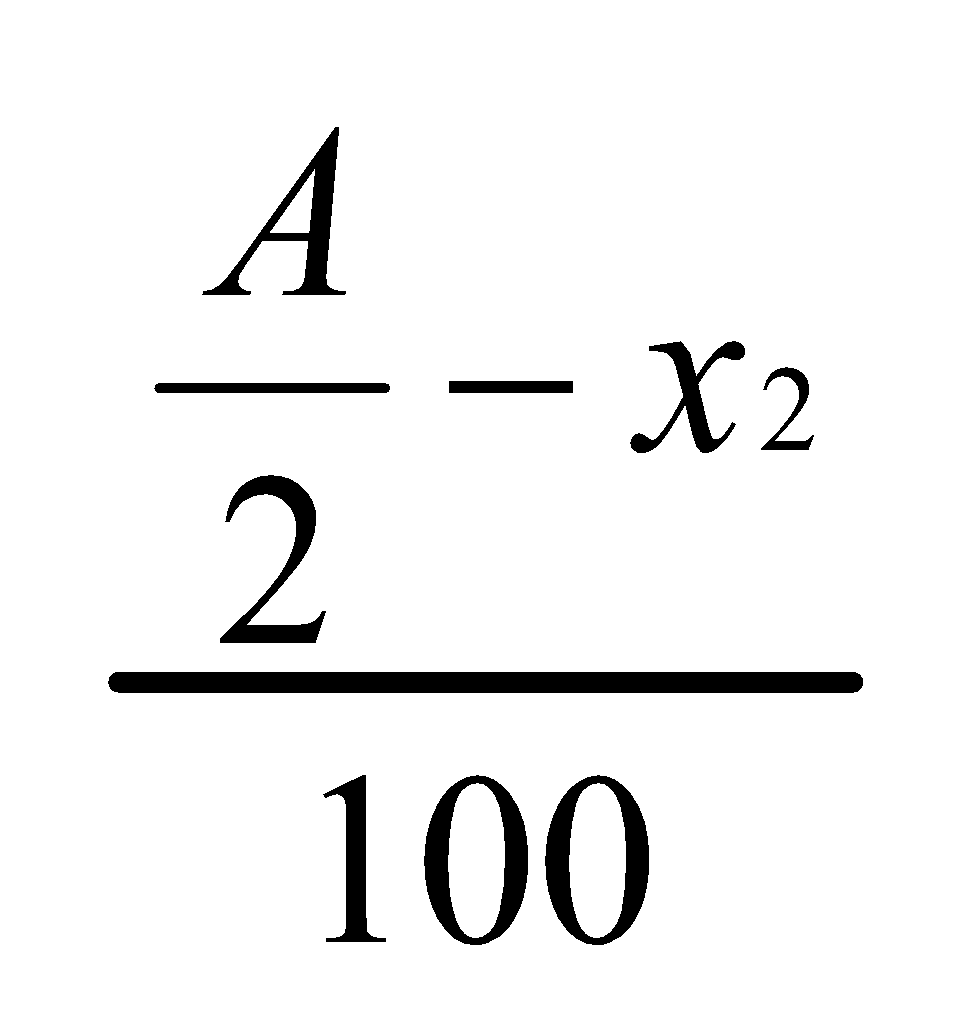
= 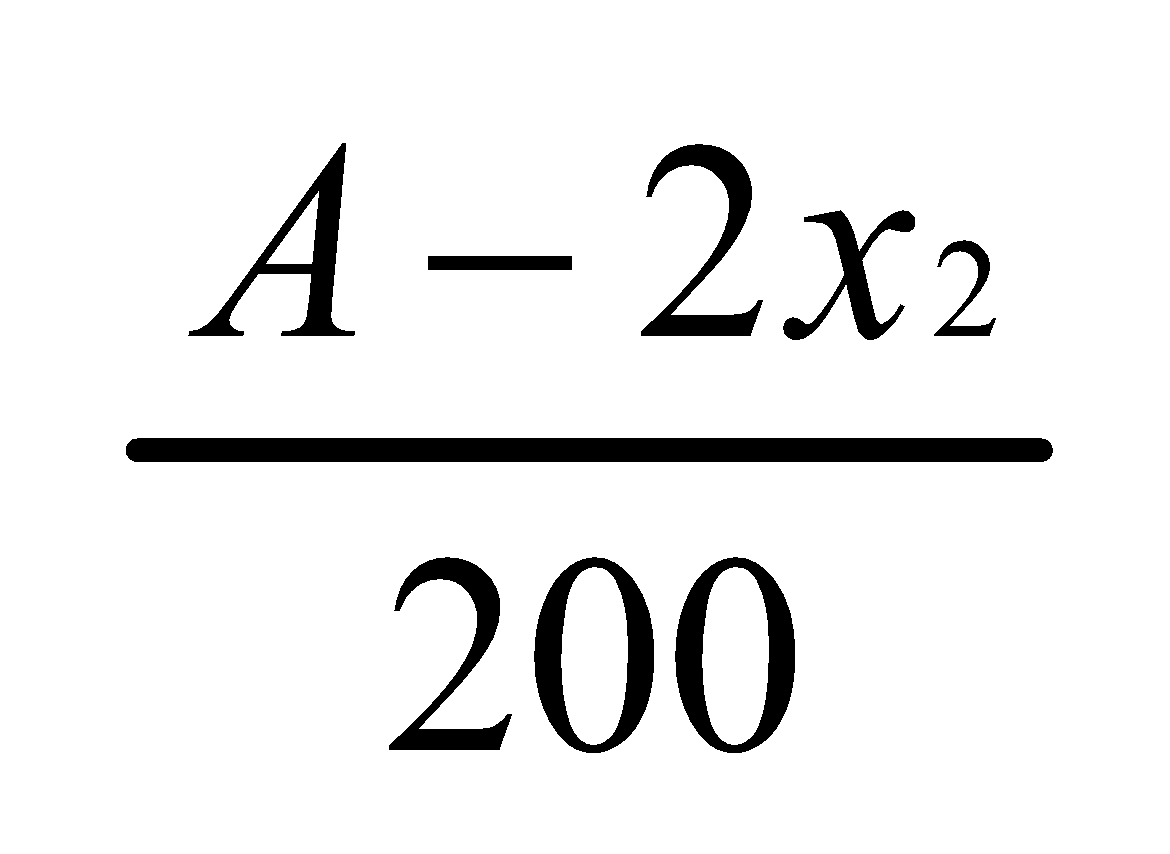
According to the Distribution law 
Thus,  Where K=2
Where K=2
Hence, 
Thus 25% of substance is extracted.
Now the total percent of substance is extracted by using two 50 ml portions of the solvent is
= (50+25) %
=75%
It is greater than 66 percent obtained by using 100 ml solvent in one lot.
Hence multiple extraction is more efficient than simple extraction-proved.
(2) Partition Chromatography
This is a modern technique of separating a mixture of small amounts of organic materials.
(3) Desilverization of Lead (Parke’s Process)
(4) Confirmatory Test for Bromide and Iodide
(5) Determination of Association
(6) Determination of Dissociation
(7) Determination of Solubility
(8) Deducing the Formula of a Complex Ion (I3 )
(9) Distribution Indicators
Slide Share Link:
Direct download link:
Get the Power Point Slides
Slide Share Link:
Watch Video Tutorials
Software Help
Hello! may I know your reference book on this. Thank you
ReplyDelete