Laboratory Manual
Course: 410
Subject: Practical: Medicinal Chemistry-II

Course: 410
Subject: Practical: Medicinal Chemistry-II
Prepared By
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Rajshahi-6205, Bangladesh
Available at
Essential Pharma documents
www.pharmacydocs.blogspot.com
Essential Pharma documents
www.pharmacydocs.blogspot.com
INDEX
Serial No.
|
Date
|
Name of the experiment
|
Page No.
|
01
|
10.10.11
|
Synthesis of Paracetamol.
|
2 – 5
|
02
|
12.10.11
|
Synthesis of acetanilide.
|
6 – 7
|
03
|
15.10.11
|
Synthesis of Esters.
|
8 – 10
|
Experiment No. 01
|
Date: 10.10.11
|
Name of the experiment: Synthesis of Paracetamol.
| |
Principle:
Paracetamol is an analgesic and antipyretic that may be conveniently prepared in the laboratory from para-aminophenol. Para-aminophenol is readily acylated with acetic anhydride to give paracetamol.
Reaction:
Mechanism of action:
The amino group is more readily acetylated than the phenolic hydroxyl group.
The following steps are involved in the formation of paracetamol:
Step-1: First step involve the formation of acetylium ion. The reaction enhanced by proton donation by concentrated acid.
Step-2: Amino group of para-aminophenol dissociates as follows:
Step-3: Acetylium ion attacks the anion at nitrogen atom to form para-acetyl aminophenol or paracetamol.
Reagents:
- Para-aminophenol 4. Concentrated H2SO4
- Acetic anhydride 5. Distilled water
- Alcohol
Apparatus:
- Beaker 5. Funnel
- Filter paper 6. Stirrer
- Water bath 7. Glass tube
- Measuring cylinder 8. Vacuum pump
Procedure:
- 5.5 gm of para-aminophenol and 15 ml of distilled water were taken in a 250 ml conical flask.
- 8 ml of acetic anhydride was added to it and was stirred. The reaction was stirred vigorously and then it was warmed on a water bath at 40-50°C for 5 minutes. The volume of reaction mixture was decreased by heating on water bath. After that, 1-2 drops of conc. H2SO4 was added in the solution.
- After 10 minutes when solid was dissolved, it was then cooled in refrigerator until the crystals were appeared and was filtered by a vacuum pump. The solid portion was acetyl derivative which was washed with little cold water.
- It was then recrystallized from 75 ml hot water and 15 ml alcohol. The final yield was then filtered by vacuum pump and was dried upon filter paper in the hot air oven. The dried solid crystals were taken and weighed.
Calculation:
Molecular weight of para-aminophenol = 109
Molecular weight of paracetamol = 151
109 gm of para-aminophenol yields 151 gm paracetamol
1 gm of para-aminophenol yields = 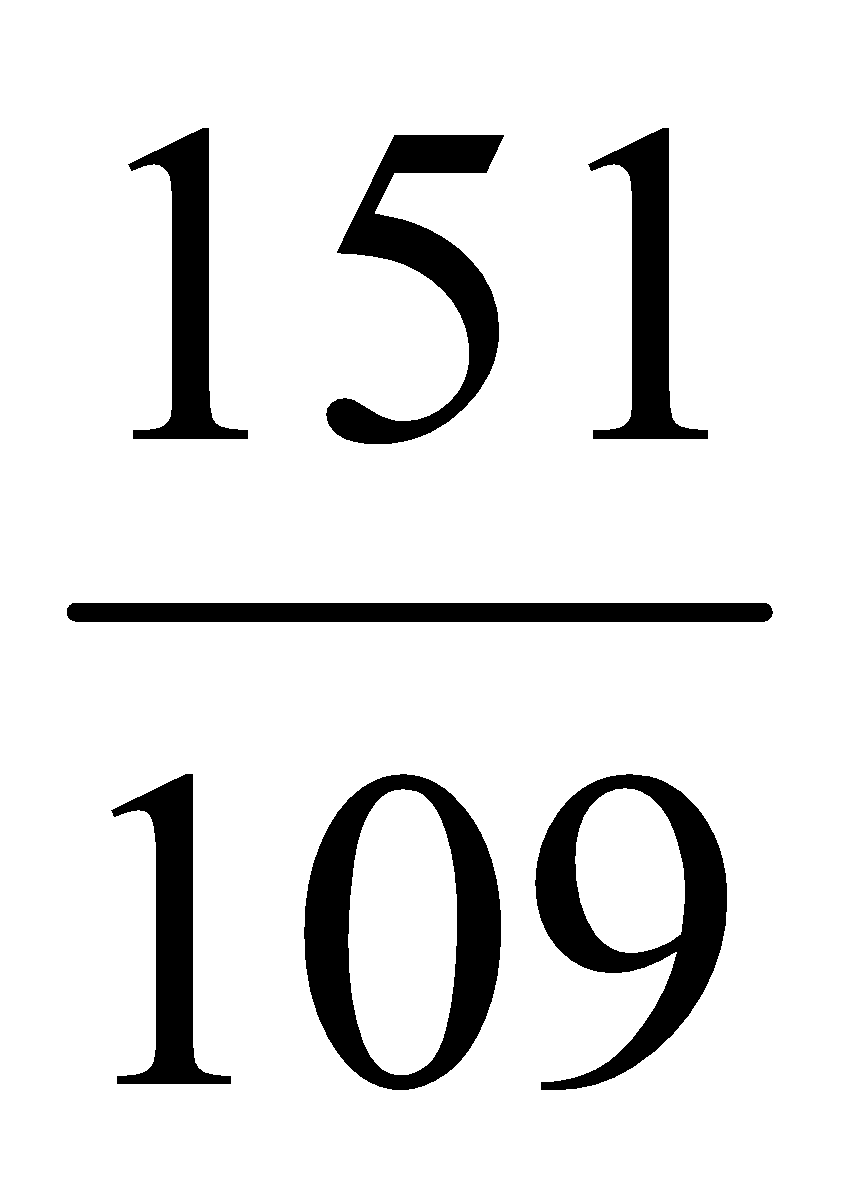 gm paracetamol
gm paracetamol
Thus, gm of para-aminophenol yields = ⎯⎯⎯⎯⎯⎯ gm paracetamol
= gm paracetamol
Now, upon drying the weight of paracetamol crystal was = gm
Practical yield weight of aspirin
% of paracetamol yields =  × 100
× 100
= ⎯⎯⎯⎯⎯⎯⎯ × 100
=
Result:
The percentage yield of paracetamol from the experiment was =
Determination of melting point: The synthesized paracetamol showed the melting point at 169°C.
Comment:
The percent yield of paracetamol was the normal.
It may be due to the following reasons:
- A portion of the supplied sample may remain during unreacted or byproduct may be formed by various side reactions.
- A portion of the product may be lost during washing and drying.
- Weight variation may be occurred due to instrumental defect.
- Amount of reagent and starting materials taken may not be exact.
- Supplied reagent and starting material may not be pure.
- Cooling or heating may not be done properly.
- Filter paper may absorb a portion of crystal.
Properties of paracetamol:
- Crystaline powder and after purification it was white color.
- Slightly bitter taste and odorless.
- Melting point range is 169-170°C.
- It is soluble in 7 parts of ethanol and 70 parts of water.
Indication: Paracetamol has both analgesic and antipyretic actions.
- As analgesic, paracetamol is used for the treatment of:
- Headache
- Toothache
- Dysmenorrhoea
- Neuralgia
- Rheumatic pain
- Arthralgia
.
- As antipyretic, paracetamol is used for the treatment of influenza. It is often used in place of aspirin in order to avoid possibility of Roy’s syndrome.
Side effects:
- Early:
- Nausea iii. Anorexia
- Vomiting iv. Abdominal pain
- Delayed:
- Hepatic necrosis v. Skin rash
- Renal tubular necrosis vi. Pancytopenia
- Hypoglycemic coma vii. Urticaria
- Leucopenia
Dose:
Single dose: 500 mg or 1 gm dauily. Dose should not be exceeding 4 gm per day.
Dose for children is about 30 mg/kg body weight.
Precaution:
A doctor should consult before administration of paracetamol to a child age less than 3 years. In children under 12 years, dose should not exceed 1-2 gm. Single dose should not be given at an interval of less than 4 hours.
Dosage form:
- Tablet ⇒ Elixir
- Solution ⇒ Suspension
- Syrup ⇒ Capsule
Contraindication:
- Hypersensitivity to paracetamol ⇒ Liver disease
- Glucose-6-phosphate dehydrogeneous deficiency ⇒ Kidney disease
Market preparation:
Trade name
|
Manufacturer name
| |
Napa
|
Beximco
| |
Apa
|
Opsonin
| |
Ace
|
Square
| |
Fast
|
Acme
| |
Reset
|
Incepta
| |
Aceta
|
Biopharma
| |
Zerin
|
Jayson
| |
Parapyrol
|
GSK
| |
ATP
|
General
| |
Remalgin
|
Reman drug
| |
Xpa
|
Aristopharma
| |
Experiment No. 02
|
Date: 12.10.11
| |
Name of the experiment: Synthesis of acetanilide.
| ||
Principle:
Acetanilide can be synthesized from aniline by acetylation in presence of acetic anhydride. Acetic acid and zinc is used as catalyst (as reducing agent).
Reaction:
Apparatus:
- Round bottom flask vi. Pipette
- Reflux condenser vii. Suction pump
- Conical flask viii. Glass rod
- Water bath ix. Hot air oven
- Beaker x. Filter paper
Reagents:
- Aniline
- Acetic anhydride
- Distilled water
- Glacial acetic acid
- Zinc dust
- Ethanol
- Conc. H2SO4
Purposes:
- To synthesize an acetanilide by reaction of aniline and acetic anhydride.
- To purify acetanilide by crystallization method from water.
Procedures:
- 2.33 gm (2.5 ml) of aniline was placed to a 150–250 ml round bottom flask.
- 3.06 gm acetic anhydride (2.5 ml) was gradually added to a reaction mixture followed by glacial acetic acid (1.44 gm or 1.5 ml) and zinc dust (0.025 mol = 1.65 gm) or sodium metabisulfite.
- The reaction mixture was heated at 50–60° C until the mixture was dried.
- The reaction mixture was cooled at room temperature and there was added 75 ml ice water in it.
- The precipitation of crude acetanilide was collected by suction pump and dried in hot air oven.
- Recrystallization: The crude acetanilide was dissolved in 5 ml of ethanol. Then 100 ml boiled water was added in the mixture gradually. The volume of solvent must be added till to turbidity and further heated to clear off the solution.
- The solution was cooled at room temperature or in an ice water bath, the crystal was filtered at pump and dried in the hot air oven.
Calculation:
Molecular weight of aniline M1 = 93
Molecular weight of acetanilide M2 = 135
Taken amount of aniline W1 = 2.33 gm
Found amount of acetanilide W2 = gm
% yield of acetanilide =  × 100
× 100
= ⎯⎯⎯⎯⎯ × 100
= %
Result:
The yield of acetanilide was % having the melting point 114° C.
Precaution:
Aniline and acetanilide are toxic chemicals. So care should be taken to avoid contact with them.
Mode of action:
Since acetanilide is in the same class of drugs of acetaminophen or paracetamol, it shows analgesic and antipyretic activity. It produces analgesic action by elevation of the pain threshold and antipyresis through the action on the hypothalamic heat regulating centre.
Pharmaceutical uses:
- Acetanilide has analgesic and antipyretic properties. It is in the same class of drug as paracetamol.
- It is used as an inhibitor in hydrogen peroxide and use to stabilize cellulose ester.
- It is used as a precursor in penicillin synthesis and other pharmaceuticals and its intermediates.
Experiment No. 03
|
Date: 15.10.11
|
Name of the experiment: Synthesis of Esters.
| |
Introduction:
The esters are a group of organic compounds best known for their interesting odours. Many perfumes and artificial flavorings are esters. Esters are formed when a carboxylic acid reacts with an alcohol in the presence of a strong acid. A general equation for the formation of esters is:
The R and R' represents alkyl groups such as methyl, ethyl, or propyl. The esters are named after the compounds from which they are formed. The first part of the name comes from the alcohol and the second part of the name comes from the carboxylic acid. Thus when ethyl alcohol (ethanol) combines with acetic acid, the resulting ester is named ethyl acetate.
The synthesis of an ester must be done in the presence of an acid in order to push the reaction closer to completion. The reaction can be reversed by adding a strong base, such as NaOH. The acid that you will be using as a catalyst in this experiment is sulfuric acid.
Many of the aromas of natural fruits and flowers are due to simple esters. Octyl ethanoate has the odor of oranges, while apricots owe their characteristic aroma to pentyl butanoate.
Mechanism for reaction for acid catalyzed esterification:
Step 1: An acid/base reaction. Protonation of the carbonyl makes it more electrophilic.
Step 2: The alcohol O functions as the nucleophile attacking the electrophilic C in the C=O, with the electrons moving towards the oxonium ion.
Step 3: Deprotonation of the alcoholic oxygen.
Step 4: Protonation of –OH group of compound makes it good leaving group.
Step 5: The electrons of adjacent oxygen helps to leaving the group as a water molecule.
Step 6: Deprotonation of the oxonium ion reveals the carbonyl in the ester product.
Apparatus:
- Beaker (250 ml) vii. Full face shield
- 2 test tubes viii. Centigram balance
- Thermometer ix. Glazed paper
- Test tube rack x. Plastic gloves
- Safety goggles xi. Graduated cylinder (10ml)
- Dropper pipette xii. Hotplate
Reagents:
- Methanol iv. Salicylic acid
- Ethanol v. Concentrated sulfuric acid
- Glacial acetic acid
Procedure:
- Two test tubes were taken and labeled them as A and B.
- Following reagent in the following ratio:
- 1 ml acetic acid 1 ml ethanol
- 1 g salicylic acid 1 ml methanol
- 4 drops of conc. sulfuric acid was carefully added to each test tube.
- About 150 ml of water was putted in a 250 ml beaker. The test tubes were placed into the water and heated the water on a hot plate to a temperature of 60°C. The test tubes were heated in the hot water bath for 15 minutes. It was ensured that, the water bath remained at approximately 60°C for that period of time.
- The test tubes were cooled for a few minutes and then immersed in an ice bath. This step was added to prevent any test tubes from cracking.
- 5 ml of distilled water was added to each of the test tubes. This step was included to dilute the ester and soften its odour.
- The odour of the contents of each of the test tubes was carefully noted. It was done by wafting the odours of the test tubes toward the nose. The observations were documented in the table.
Required Combinations:
- Methyl alcohol + acetic acid, benzole acid, salicylic acid
- Ethyl alcohol + acetic acid, benzoic acid, salicylic acid
Result:
Test tube
|
Alcohol
|
Carboxylic acid
|
Ester Produced
|
Odor of Ester
|
1
|
Ethyl alcohol
|
Acetic acid
|
Ethylacetate
|
Fingernail Polish Remover
|
2
|
Ethyl alcohol
|
Benzoic Acid
|
Ethylbenzoate
|
Fruity
|
3
|
Methyl alcohol
|
Salicylic acid
|
Methyl Salicylate
|
Wintergreen
|
Safety Precautions:
- Concentrated sulfuric acid is a strong oxidizing agent. It will start a fire if mixed incorrectly with any of the alcohol or other acids used in this experiment. Use exactly as directed.
- All of the liquid acids used in this experiment are corrosive to skin, eyes and clothing. While working on this experiment, ware safety goggles, full face shield, gloves and lab apron. Wash spills and splashes off the skin and clothing immediately, using plenty of water.
- The alcohol and the organic acids used in this experiment are all flammable. Be sure all burners and other flames in the laboratory are extinguished before starting the experiment.
- The detection of odours should be done cautiously. Breathing the vapors of some of these esters can cause sore throat, dizziness, headache and drowsiness. The test tube should be held about 30 cm away and 15 cm below the nose. Waft the odor toward the nose, sniffing cautiously, once or twice. Should not breathe deeply while sniffing.
No comments:
Post a Comment