Laboratory Manual
Course: 410
Subject: Practical - Pharmacology-III

Course: 410
Subject: Practical - Pharmacology-III
Prepared By
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Rajshahi-6205, Bangladesh
Available at
Essential Pharma documents
www.pharmacydocs.blogspot.com
Essential Pharma documents
www.pharmacydocs.blogspot.com
INDEX
Sl. No.
|
Date
|
Name of the experiment
|
Page No.
|
01
|
19.09.11
|
Estimation of glucose concentration in blood after oral administration of glucose.
|
2–8
|
02
|
20.09.11
|
Estimation of glucose concentration in blood at fasting condition.
|
9–13
|
Experiment No. 01
|
Date: 19.09.11
|
Name of the experiment: Estimation of glucose concentration in blood after oral administration of glucose.
| |
Introduction:
Glucose is the primary source of energy for the body's cells. The mean normal blood glucose level in humans is about 80–120 mg/dl (4.4–6.1 mmol/L). The human body naturally tightly regulates glucose levels in blood as a part of metabolic homeostasis.
Sugar levels in blood outside the normal range may be an indicator of a medical condition.
Hypoglycemia: Hypoglycemia is a condition is which blood sugar level is present below the normal, that is, below 80 mg/dL. Symptoms may include lethargy, impaired mental functioning, irritability, shaking, twitching, sweating and loss of consciousness, weakness in arm and leg muscles. Hypoglycemic symptoms are relieved by the administration of glucose.
Hyperglycemia: It is a condition in which blood sugar level increases above the normal i.e. 120 mg /dL. Long-term hyperglycemia causes many of the long-term health problems associated with diabetes, including eye, kidney, heart disease and nerve damage. When the blood sugar level exceeds the renal threshold (180 mg /dL) sugar appears in urine.
Diabetes mellitus: Diabetes is a metabolic disorder characterized by hyperglycemia due to deficiency of insulin. This is a disease in which body does not properly control the amount of sugar in blood. As a result the level of sugar in the blood becomes too high, that is, 7 m mol/L in fasting condition and after 2 hours of diet is more that 11.1 m mol/L.
Classification: There are two main types of diabetes mellitus.
- Type – 1: Insulin dependent diabetes mellitus (IDDM) or juvenile onset diabetes.
- Type – 2: Non-insulin dependent diabetes mellitus (NIDDM) or maturity onset diabetes.
Symptoms of Diabetes Mellitus:
- Hyperglycemia
- Polyuria
- Polyphagia
- Polydipsia
- Weight loss
- Weakness
- Itching
viii. Increased frequency of infections
ix. Fasting blood glucose is higher than 126 mg/100ml.
Risk factors of diabetes: Diabetes mellitus can causes serious hyperglycemia and if it left untreated, it can result in:
- Retinopathies iv. Nephropathy
- Glaucoma v. Cardiovascular complication
- Neuropathies vi. Increased incidence of toxemia of pregnancy
Units:
The international standard way of measuring glucose levels in blood are in terms of a molar concentration, measured in mmol/L. In the United States, mass concentration is measured in mg/dL.
Since the molecular weight of glucose C6H12O6 is about 180 g/mol, for the measurement of glucose, the difference between the two scales is a factor of 18, so that 1 mmol/L of glucose is equivalent to 18 mg/dL.
Glucose level in blood at different stages:
Normal range = 80 – 120 mg / 100 (4.4–6.1 mmol/L) ml
Post prandial glucose level in blood:
72–126 mg/dl (4–7 mmol/L): Normal
> 126–180 mg/dl (7–10 mmol/L)): Suggestive of diabetes
> 180 mg/dl (10 mmol/ L): Almost certainly diabetes
|
Fasting blood glucose level:
65–110 mg/dl (3.6–6.1 mmol/L): Normal
> 110–160 mg/dl (6.1–9 mmol/L): Suggestive of diabetes
> 180 mg/dl (10 mmol/L): Almost certainly diabetes
|
Measurement of glucose level in blood:
Glucose is measured in whole blood, plasma or serum. Historically, blood glucose values were given in terms of whole blood, but most laboratories now measure and report the serum glucose levels. Because red blood cells (erythrocytes) have a higher concentration of protein (e.g., hemoglobin) than serum, serum has a higher water content and consequently more dissolved glucose than does whole blood.
Measurement techniques:
Two major methods have been used to measure glucose.
- The first one is a chemical method exploiting the nonspecific reducing property of glucose in a reaction with an indicator substance that changes color when reduced. Since other blood compounds also have reducing properties (e.g., urea), this technique can produce erroneous readings in some situations (5 to 15 mg/dl has been reported). In this method, different oxidizing agent (e.g., Fehling solution, Arsenic molybdate, Alkaline ferricyanide etc.) are used.
- The more recent technique is the enzymatic method in which specific enzymes to glucose are used. The two most common employed enzymes are glucose oxidase and hexokinase. This method is less susceptible to give erroneous reading.
Blood glucose laboratory tests:
- Fasting blood sugar test (FBS)
- Urine glucose test
- Two-hour postprandial blood sugar test (2-h PPBS)
- Intravenous glucose tolerance test (IVGTT)
- Glycosylated hemoglobin (HbA1C)
- Self-monitoring of glucose level via patient testing
Oral glucose tolerance test: A screening test for diabetes mellitus, in which plasma glucose levels are measured after the patient consumes an oral glucose load. Plasma glucose levels between 140−199 mg/dl suggest impaired glucose tolerance and plasma glucose levels when exceed 200 mg/dl after 2 hours of drinking a 75 g glucose load suggest diabetes mellitus.
It is regarded as the gold standard of clinical tests of the insulin / glucose control system, but is difficult to administer, requiring much time and repeated blood tests.
Different factors affecting the glucose level in experiment:
- Collection of blood in clot tubes for analysis permits the metabolism of glucose in the sample by blood cells until separated by centrifugation. As a result glucose concentration in sample may reduce.
- Higher than normal amounts of white or red blood cell counts can lead to excessive glycolysis in the sample, with substantial reduction of glucose level if the sample is not processed quickly.
- Ambient temperature at which the blood sample is kept prior to centrifuging and separation of plasma/serum also affects glucose levels.
- The glucose level in sample may also be reduced due to the use of normal glass centrifuge tube. This loss of glucose can be prevented by using Fluoride tubes (i.e., gray-top) since fluoride inhibits glycolysis.
- Error rates for blood glucose measurements systems vary, depending on laboratories and on the methods used. Spectrophotometry techniques can be biased by color changes in cell (from airborne or finger borne contamination) or interference (e.g., tinting contaminants) with light source or the light sensor.
So, these factors must be carefully considered during the experiment to find out accurate result.
Principle:
The glucose of blood sample determination is based on the reaction of glucose with Fehling solution. Fehling solution is mild oxidizing agent and easily oxidizes the reducing sugar such as glucose, present in blood. Blood also contain a small glucoronate which may give rise to Cu2O formation but here mainly blood glucose is considered.
Fehling solution contain Cu2+ ions and is prepared by adding Fehling’s-A solution containing CuSO4 to Fehling’s-B solution containing NaOH and Rochelle salt (Na-K tartrate).
During oxidation to aldehyde to acid, the Cu2+ ions reduced to Cu+ ions which are precipitated as red color of Cu2O.
R–CHO + 2Cu2+ + 3OH– ⎯→ R–COO– + 2Cu+ + 2H2O
2Cu2+ + 2OH– ⎯→ Cu2O ↓ + H2O
The color of the blood sample (after reduction with Fehling solution) is adjusted with Arsenic molybdate to form a color complex.
Arsenic molybdate + Cu2O ⎯→ Color complex (soluble)
The absorbance of color complex is measured by spectrophotometer at 520 nm wavelength (λmax). By plotting the absorbance value of blood glucose sample on the calibration curve, we can easily calculate the blood glucose concentration.
Reagents:
- Arsenic molybdate
- 10% Na-tungstate
- Anticoagulant (Na-oxalate)
- Fehling solution (A:B = 25:1)
- Glucose
- 2/3 N H2SO4
Function of the reagents:
- Arsenic molybdate: It is used to form complex with the solution so that, it can give proper absorbance at maximum wavelength.
- Na-tungstate: It is used to precipitate the proteins and cell contents present in the blood.
- Anticoagulant (Na-oxalate): It is used to prevent the clotting of blood within the test tube.
- Fehling solution: It consists of two components: Fehling’s A (a copper sulphate solution) and Fehling’s B (a solution of potassium sodium tartrate and sodium hydroxide). It is used to form color complex with the solution so that, it can give proper absorbance at maximum wavelength.
- Sulfuric acid: It is used to precipitate the proteins and cell contents present in the blood as we find the clear supernatant.
Apparatus:
- Test tube
- Graduated pipette
- Water bath
- Volumetric flask
- Measuring cylinder
- Spectrophotometer
- Centrifuge tube
viii. Centrifuge machine
Preparation of standard glucose solution (Stock solution):
- gm of glucose was taken in 100 ml volumetric flask and volume was adjusted to 100 ml by dissolving glucose and adding distilled water. Therefore, the concentration is 1 mg/ml or 1000 µg/ml.
Preparation of 20 µg/ml, 40 µg/ml, 60 µg/ml, 80 µg/ml, 100 µg/ml solution:
5 volumetric flasks were taken, washed and marked respectively with concentration as above. Then 2 ml, 4 ml, 6 ml, 8 ml and 10 ml of stock solution were taken in corresponding volumetric flask and adjusted to mark with distilled water.
Preparation of sample solution:
- 3 ml of blood sample was withdrawn into a test tube containing 1 ml Na-oxalate anticoagulant.
- 0.5 ml blood, 0.5 ml Na-tungstate, 0.5 ml 2/3N H2SO4 and 8.5 ml distilled water were taken in a centrifuge tube and was centrifuged for 5 min at 400 rpm.
- Then 0.5 ml of supernatant serum was taken in another test tube and 0.5 ml Fehling solution was added in it and mixed well.
Procedure
- 7 test tubes were taken and labeled as blank, 20 µg/ml, 40 µg/ml, 60 µg/ml, 80 µg/ml, 100 µg/ml and sample.
- 0.5 ml distilled water (for blank), 0.5 ml of each standard solution and 0.5 ml supernatant fluid were taken by 1 ml pipette into corresponding test tubes and 0.5 ml Fehling solution was added in each test tube.
- Then test tubes were heated in water bath for 30 minutes and cooled.
- 0.5 ml Arsenic molybdate and 8.5 ml distilled water were added in each test tube and mixed well.
- The absorbance was taken in spectrophotometer (UV) at 520 nm wavelength for all solution.
- Finally a standard point calibration curve for glucose was obtained by plotting absorbance versus concentration. Then the concentration of supplied sample was calculated from standard curve for respective absorbance.
Data:
For glucose concentration in blood after oral administration of glucose:
Concentration of solution (μg/ml)
|
Absorbance (λmax = 520 nm)
|
Blank
| |
20
| |
40
| |
60
| |
80
| |
100
| |
Sample
|
Calculation:
Concentration of sample from curve is = µg/ml
Dilution factor:
0.5 ml blood is taken into 10 ml of solution
∴ Dilution factor = 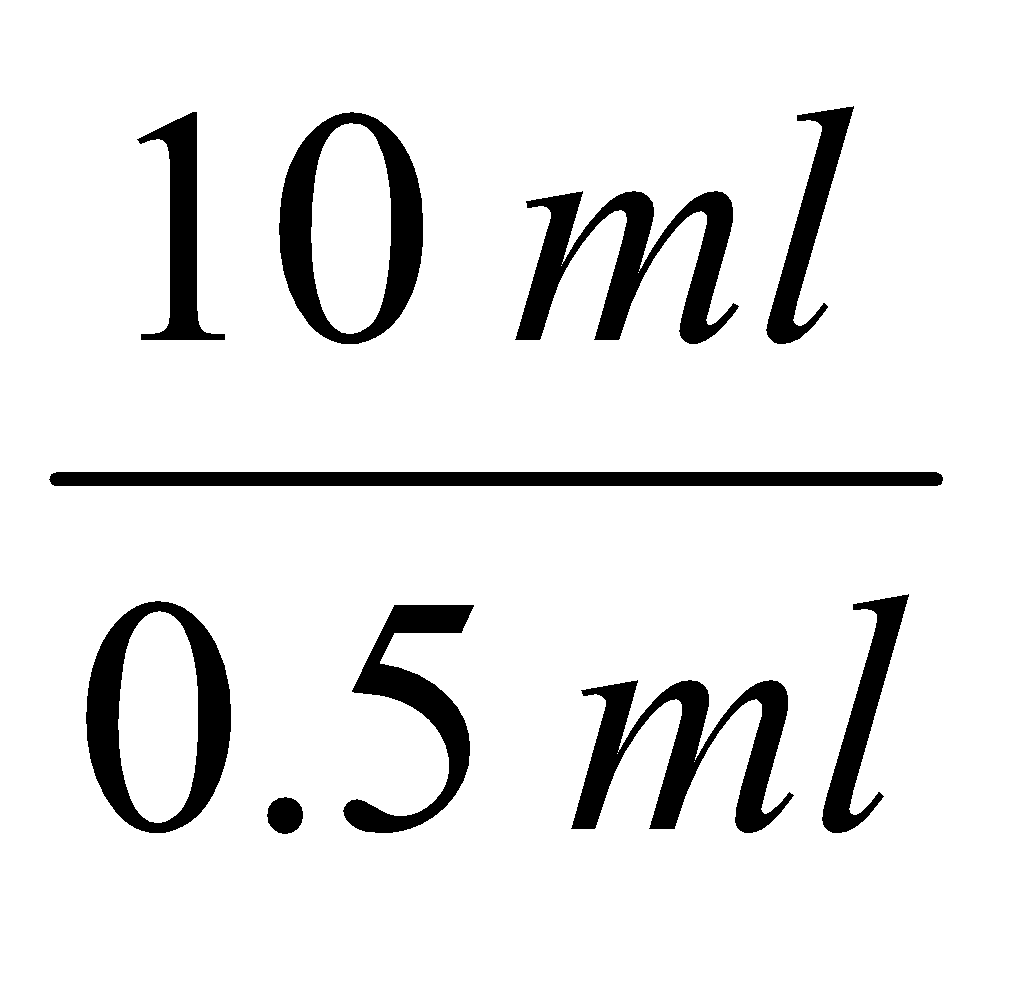 = 20
= 20
Actual concentration of glucose in blood = µg/ml
Result:
After experiment from the calibration curve it was found that, the concentration of blood glucose after oral administration of glucose was µg/ml.
Comment:
Experiment No. 02
|
Date: 20.09.11
|
Name of the experiment: Estimation of glucose concentration in blood at fasting condition.
| |
Introduction:
Glucose is the primary source of energy for the body's cells and blood lipids (in the form of fats and oils) are primarily a compact energy store. Glucose is transported from the intestines or liver to body cells via the bloodstream and is made available for cell absorption via the hormone insulin, produced by the body primarily in the pancreas.
The mean normal blood glucose level in blood in humans is about 80–120 mg / 100 ml (4.4–6.1 mmol/L). However, this level fluctuates throughout the day. Glucose levels are usually lowest in the morning, before the first meal of the day (termed the fasting level) and rise after meals for an hour or two.
The human body naturally tightly regulates glucose levels in blood as a part of metabolic homeostasis. Sugar levels in blood outside the normal range may be an indicator of a medical condition.
Measurement of fasting glucose level in blood:
The fasting glucose level in blood is a much poorer screening test because of the high variability of the experimental conditions such as the carbohydrate content of the last meal and the energy expenditure between the last meal and the measurement.
The fasting glucose level in blood is measured after a fast of 8 hours, is the most commonly used indication of overall glucose homeostasis, largely because disturbing events such as food intake are avoided. Abnormalities in these test results are due to problems in the multiple control mechanism of glucose regulation.
Conditions affecting glucose levels are shown in the table below:
Table: Causes of abnormal glucose levels in blood.
Persistent hyperglycemia
|
Transient hyperglycemia
|
Persistent
hypoglycemia
|
Transient hypoglycemia
|
Diabetes mellitus
|
Acute alcohol ingestion
| ||
Adrenal cortical hyperactivity Cushing's syndrome
|
Severe liver disease
|
Adrenal cortical insufficiency Addison's disease
| |
Acute stress reaction
|
Severe liver disease
| ||
Several glycogen storage diseases
| |||
Ectopic insulin production from tumors
|
Hereditary fructose intolerance
|
Principle:
The simplest plasma or blood test for glucose used in establishing the diagnosis of diabetes is fasting blood glucose test. The blood sample is drawn from person in morning prior to breakfast (usually overnight fast).
The normal fasting plasma glucose is usually set in between 3.6 mmol/L (65 mg/dl) and 6.1 mmol/L (110 mg/dl). The diagnosis of diabetes may be confirmed in patient with two or more fasting plasma glucose level that are elevated above 7.8 mmol/L (140 mg/dl).
The glucose of blood sample determination is based on the reaction of glucose with Fehling solution. Fehling solution is mild oxidizing agent and easily oxidizes the reducing sugar such as glucose, present in blood. Blood also contain a small glucoronate which may give rise to Cu2O formation but here mainly blood glucose is considered.
Fehling solution contain Cu2+ ions and is prepared by adding Fehling’s-A solution containing CuSO4 to Fehling’s-B solution containing NaOH and Rochelle salt (Na-K tartrate).
During oxidation to aldehyde to acid, the Cu2+ ions reduced to Cu+ ions which are precipitated as red color of Cu2O.
R–CHO + 2Cu2+ + 3OH– ⎯→ R–COO– + 2Cu+ + 2H2O
2Cu2+ + 2OH– ⎯→ Cu2O ↓ + H2O
The color of the blood sample (after reduction with Fehling solution) is adjusted with Arsenic molybdate to form a color complex.
Arsenic molybdate + Cu2O ⎯→ Color complex (soluble)
The absorbance of color complex is measured by spectrophotometer at 520 nm wavelength (λmax). By plotting the absorbance value of blood glucose sample on the calibration curve, we can easily calculate the blood glucose concentration.
Reagents:
- Arsenic molybdate
- 10% Na-tungstate
- Anticoagulant (Na-oxalate)
- Fehling solution (A:B = 25:1)
- Glucose
- 2/3 N H2SO4
Function of the reagents:
- Arsenic molybdate: It is used to form complex with the solution so that, it can give proper absorbance at maximum wavelength.
- Na-tungstate: It is used to precipitate the proteins and cell contents present in the blood.
- Anticoagulant (Na-oxalate): It is used to prevent the clotting of blood within the test tube.
- Fehling solution: It consists of two components: Fehling’s A (a copper sulphate solution) and Fehling’s B (a solution of potassium sodium tartrate and sodium hydroxide). It is used to form color complex with the solution so that, it can give proper absorbance at maximum wavelength.
- Sulfuric acid: It is used to precipitate the proteins and cell contents present in the blood as we find the clear supernatant.
Apparatus:
- Test tube
- Graduated pipette
- Water bath
- Volumetric flask
- Measuring cylinder
- Spectrophotometer
- Centrifuge tube
viii. Centrifuge machine
Preparation of standard glucose solution (Stock solution):
- gm of glucose was taken in 100 ml volumetric flask and volume was adjusted to 100 ml by dissolving glucose and adding distilled water. Therefore, the concentration is 1 mg/ml or 1000 µg/ml.
Preparation of 20 µg/ml, 40 µg/ml, 60 µg/ml, 80 µg/ml, 100 µg/ml solution:
5 volumetric flasks were taken and washed and marked respectively with concentration as above. Then 2 ml, 4 ml, 6 ml, 8 ml and 10 ml of stock solution were taken in corresponding volumetric flask and adjusted to mark with distilled water.
Preparation of sample solution:
- 3 ml of blood sample was withdrawn into a test tube containing 1 ml Na-oxalate anticoagulant.
- 0.5 ml blood, 0.5 ml Na-tungstate, 0.5 ml 2/3N H2SO4 and 8.5 ml distilled water were taken in a centrifuge tube and was centrifuged for 5 min at 400 rpm.
- Then 0.5 ml of supernatant serum was taken in another test tube and 0.5 ml Fehling solution was added in it and mixed well.
Procedure
- 7 test tubes were taken and labeled as blank, 20 µg/ml, 40 µg/ml, 60 µg/ml, 80 µg/ml, 100 µg/ml and sample.
- 0.5 ml distilled water (for blank), 0.5 ml of each standard solution and 0.5 ml supernatant fluid were taken by 1 ml pipette into corresponding test tubes and 0.5 ml Fehling solution was added in each test tube.
- Then test tubes were heated in water bath for 30 minutes and cooled.
- 0.5 ml Arsenic molybdate and 8.5 ml distilled water were added in each test tube and mixed well.
- The absorbance was taken in spectrophotometer (UV) at 520 nm wavelength for all solution.
- Finally a standard point calibration curve for glucose was obtained by plotting absorbance versus concentration. Then the concentration of supplied sample was calculated from standard curve for respective absorbance.
Data:
For glucose concentration in blood at fasting condition:
Concentration of solution (μg/ml)
|
Absorbance (λmax = 520 nm)
|
Blank
| |
20
| |
40
| |
60
| |
80
| |
100
| |
Sample
|
Calculation:
Concentration of sample from curve is = µg/ml
Dilution factor:
0.5 ml blood is taken into 10 ml of solution
∴ Dilution factor = 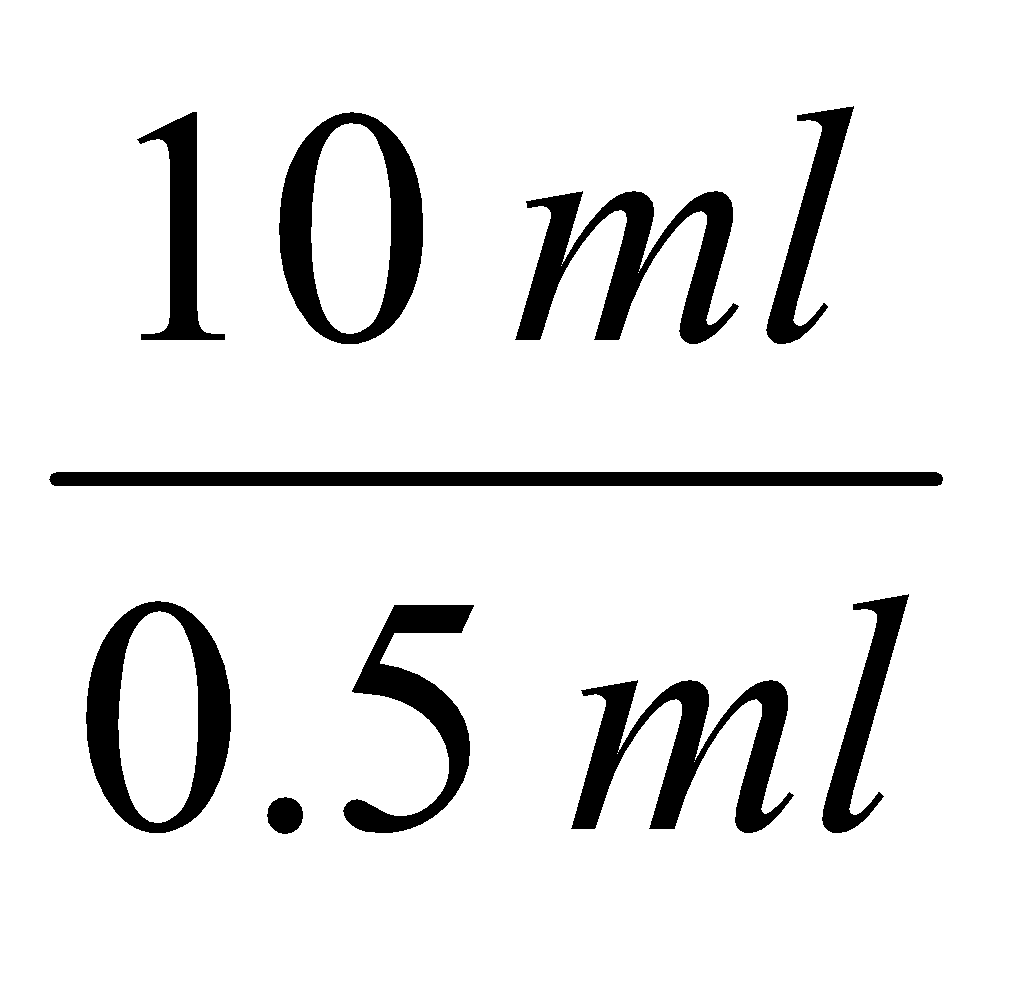 = 20
= 20
Actual concentration of glucose in blood = µg/ml
Result:
After experiment from the calibration curve it was found that, the concentration of blood glucose at fasting condition was µg/ml.
Comment:
PDF Link:
No comments:
Post a Comment