Laboratory Manual
Course: 410
Subject: Practical- Pharmaceutical Analysis-II

Course: 410
Subject: Practical- Pharmaceutical Analysis-II
Prepared By
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Rajshahi-6205, Bangladesh
Available at
Essential Pharma documents
www.pharmacydocs.blogspot.com
Essential Pharma documents
www.pharmacydocs.blogspot.com
INDEX
Sl. No.
|
Date
|
Name of the experiment
|
Page No.
|
01
|
06.02.12
|
Estimation of aspirin in a preparation by UV-spectrometric method.
|
02 – 03
|
02
|
07.02.12
|
Assay of milk of magnesia from the supplied sample.
|
04 – 07
|
03
|
08.02.12
|
Assay of ferrous fumerate from the supplied sample.
|
08 – 11
|
Experiment No. 01
|
Date: 06.02.12
|
Name of the experiment: Estimation of aspirin in a preparation by UV-spectrometric method.
| |
Principle:
UV Spectroscopic method is one of the instrumental analytical methods. It is widely used in industry research and in the clinical evaluation of many pharmaceuticals. It is relatively quick process.
The principle of UV spectrophotometer is based on the Beer-Lambert law. This law states that absorbance or optical density is directly proportional to the concentration. The law is valid only for dilute solution.
By UV spectroscopic method we can either find out the amount of drug or chemical present in the sample by taking absorbance of standard substance and sample.
Amount of the substance = 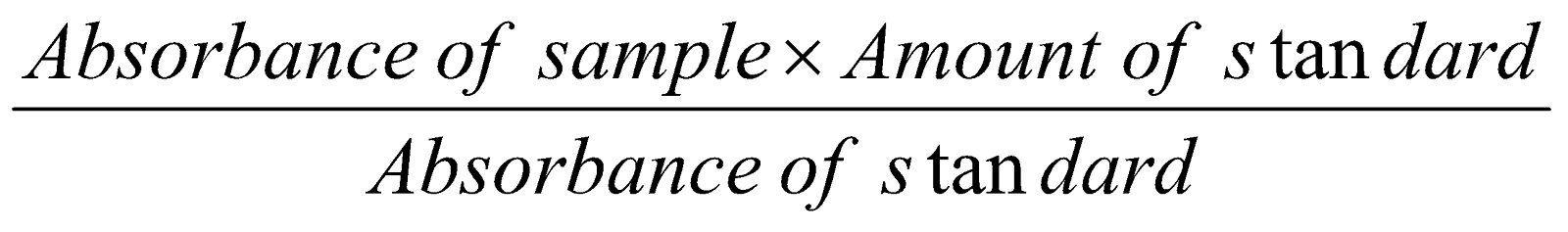
Reagent:
0.1 N NaOH solution: For preparing 500 ml 0.1 N NaOH solution, 2 gm of NaOH is taken in a 500 ml volumetric flask and the volume make up to the mark by adding distilled water.
This solution is used as blank solution and solvent for preparing standard and sample solution.
Procedure:
Preparation of standard solution:
- 150 mg of standard aspirin was dissolved in 0.1 N NaOH in a 100 ml volumetric flask and the solution was made 100 ml. No filtration was required for this solution.
- 1 ml of prepared solution was taken in a volumetric flask and made the volume 100 ml with 0.1 N NaOH. This solution was used as standard solution. The final concentration of this solution would be 15 μg/ml.
Preparation of sample solution:
- The supplied tablet was grinded into powder in a mortar and the powder was divided into two equal portions. One portion of powder which was equivalent to 150 mg of aspirin was taken in a 100 ml volumetric flask. After addition of 40–60 ml 0.1 N NaOH solution, the flask was heated in water bath at 37–40°C for 30 minutes.
- Then the solution was cooled and by adding 0.1 N NaOH solution, it was made up to 100 ml. The prepared solution was filtered.
- Again, 1 ml of filtered solution was taken in a volumetric flask and made the volume 100 ml with 0.1 N NaOH. This solution was used as sample solution. The final concentration of this solution also would be 15 μg/ml.
- Absorbance was taken for standard and sample solution at 292 nm.
Data:
Absorbance of blank solution, a =
Absorbance of standard solution, b =
Absorbance of sample solution, c =
Calculation:
Amount of sample = 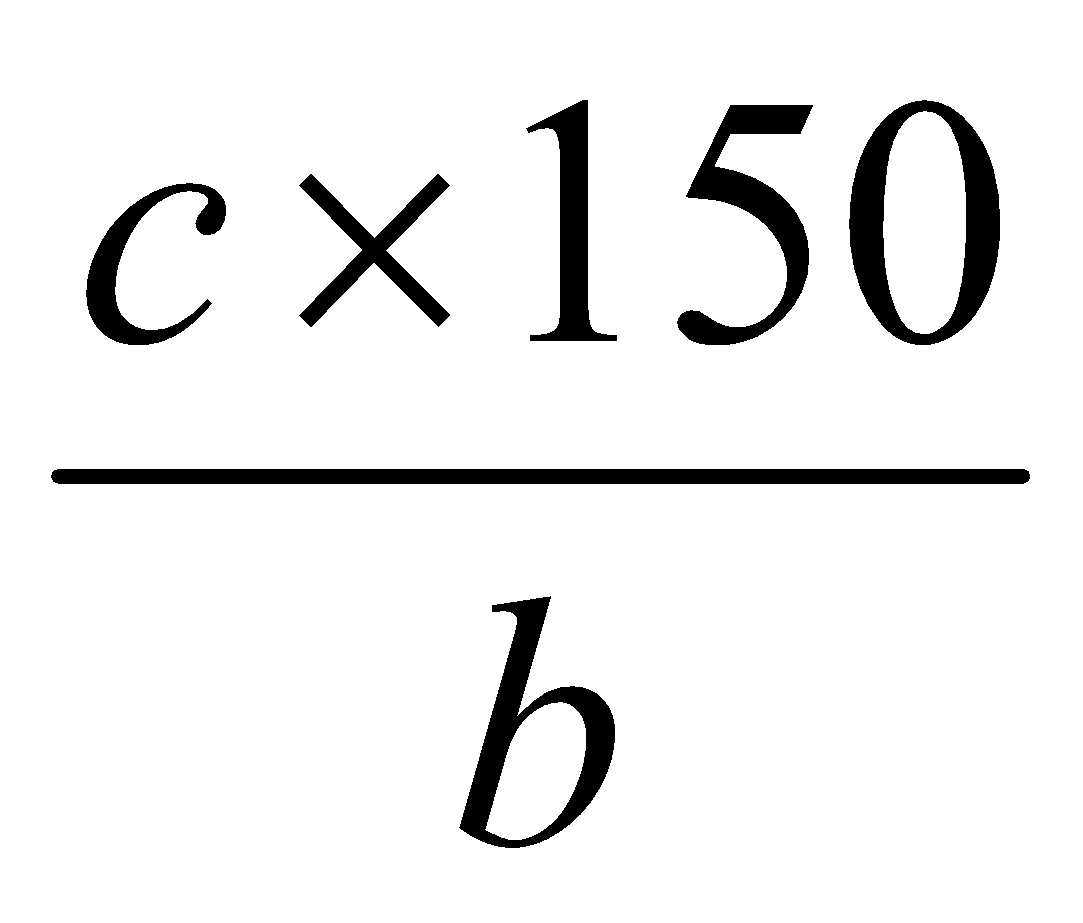 mg
mg
Tablet weight = mg
mg of aspirin powder contains = mg aspirin
” ” ” ” = ”
= (P) mg of aspirin
Potency = 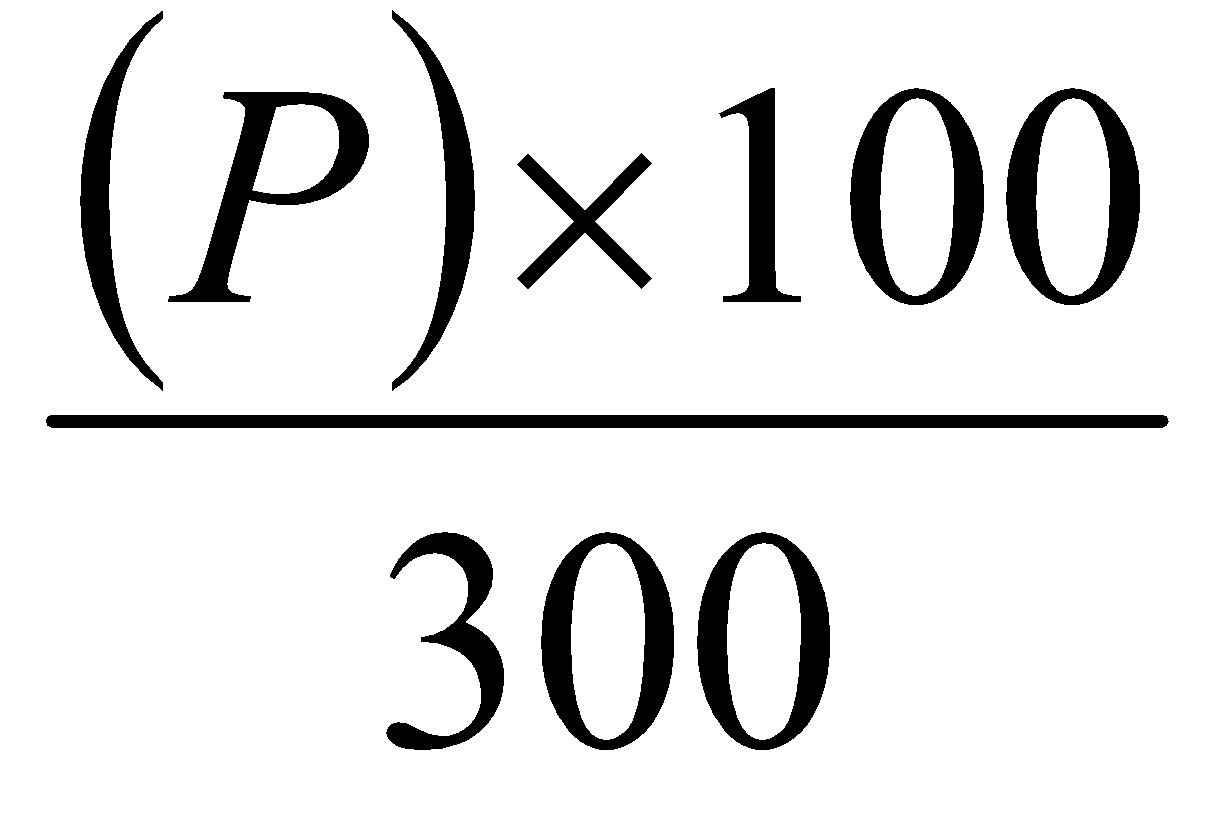
=
= %
Result:
The potency of supplied aspirin tablet was %.
Comments:
Experiment No. 02
|
Date: 07.02.12
|
Name of the experiment: Assay of milk of magnesia from the supplied sample.
| |
Principle:
Back titration is frequently used when a reaction proceeds slowly or when the substance to be assayed does not give a distinct, sharp end point with an indicator by direct titration.
Back titration or Residual titration is carried out by dissolving the substance under examination in an accurately measured quantity of standard solution in excess or by heating the reaction mixture.
To know the volume of the analyte solution, a known excess (100% to 200%) of the reagent is added. The reaction mixture is heated or kept sometimes so that the reaction is complete. Then, the unreacted reagent is determined by titration with another suitable standard reagent.
The Milk of Magnesia is dissolved in an accurately measured excess of 1 N H2SO4 solution to ensure complete neutralization of all the Mg(OH)2 with the formation of the soluble MgSO4.
The excess acid is then determined by residual titration with 1 N NaOH using methyl red as indicator.
Knowing the amount of the reagent consumed, the quantity of the analyte is calculated by stoichiometric calculation.
The reaction which takes place when the milk of magnesia is dissolved and upon residual titration are as follows:
Mg(OH)2 + H2SO4 ⎯⎯→ MgSO4 + 2H2O
H2SO4 + 2NaOH ⎯⎯→ Na2SO4 + 2H2O
1 ml of 1 N H2SO4 = 29.17 mg of Mg(OH)2
The percent of Mg(OH)2 present in the sample can be calculated from the following formula:
The USP requires that milk of magnesia contains not less than 7% and not more than 8.5% of Mg(OH)2.
Reagent and their preparation:
- 1 N 250 ml H2SO4 solution: 6.78 ml of 98.08% of conc. H2SO4 was taken into 100 ml of water in a 250 ml volumetric flask and then add water up to mark 250 ml.
- 1 N 100 ml Na2CO3 solution: 5.3 gm of Na2CO3 was taken into 7 ml of distilled water in a 100 ml volumetric flask. Finally volume was adjusted up to mark by adding distilled water.
- 1 N 250 ml NaOH solution: 10 gm of NaOH was taken into 125 ml of distilled water in a 250 ml volumetric flask. Finally volume was adjusted up to mark by adding distilled water.
Standardization of 1N H2SO4 solution by 1 N Na2CO3 solution:
- 10 ml of Na2CO3 was taken in a conical flask. 1-2 drops of methyl red indicator was added to it.
- The titration was performed using 1 N H2SO4 solution which was previously kept on burette. When the yellow color disappeared, addition of H2SO4 was stopped and red color appeared.
- The experiment was repeated for three times.
Data for standardization of H2SO4 solution:
No. of observation
|
Volume of Na2CO3 (V1 ml)
|
Volume of H2SO4 solution (ml)
|
Difference
(ml)
|
Mean volume
(V2 ml)
| |
IBR
|
FBR
| ||||
1
|
10
| ||||
2
|
10
| ||||
3
|
10
| ||||
Calculation of strength of H2SO4 solution:
We know that, V1S1 = V2S2 Here, V1 = 10 ml
S2 = 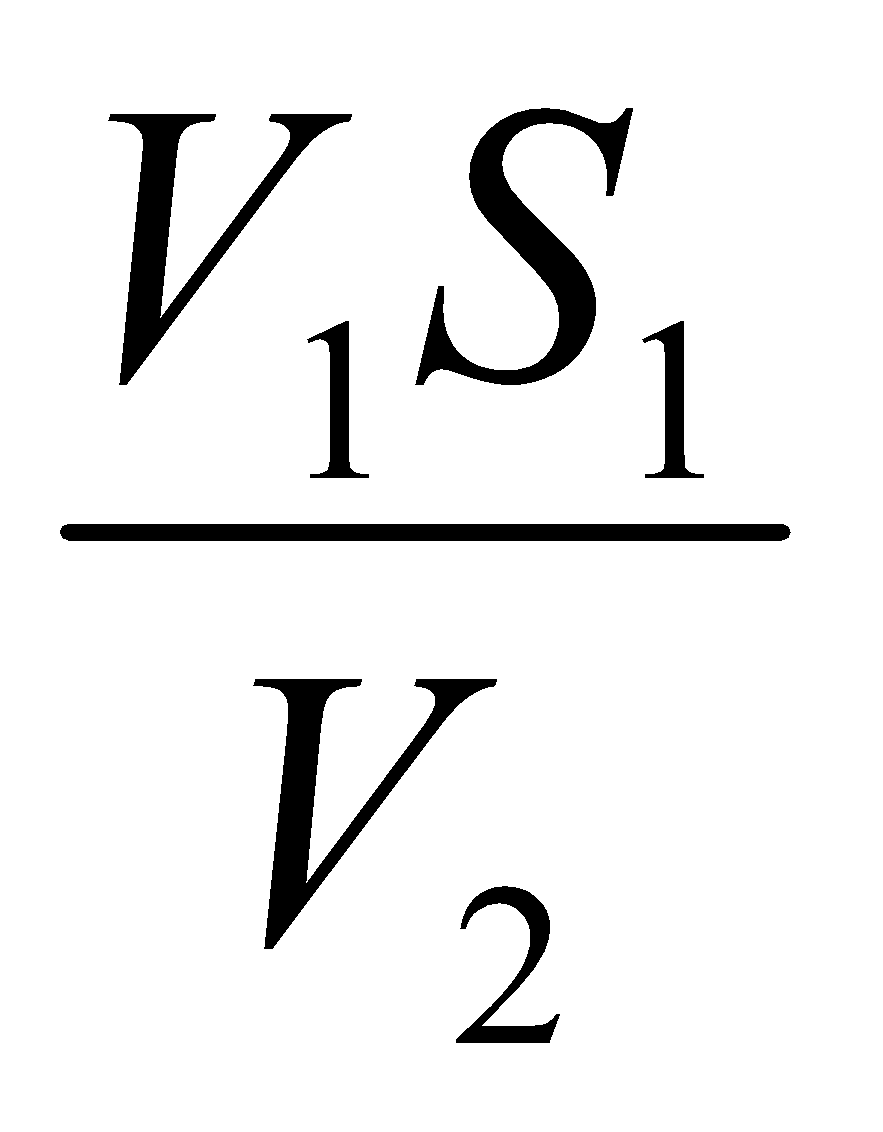 S1 = N
S1 = N
V2 = ml
= S2 = ?
= N
Standardization of 1 N NaOH by H2SO4:
- 10 ml H2SO4 was taken in a conical flask and 1-2 drops of methyl red indicator was added to it.
- The titration was performed using 1 N NaOH solution which was preciously kept on burette. When the red colour disappeared, addition of NaOH was stopped and yellow colour appeared.
- The experiment was repeated for three times.
Data for standardization of NaOH solution:
No. of observation
|
Volume of H2SO4 solution
(V1 ml)
|
Volume of NaOH solution (ml)
|
Difference
(ml)
|
Mean volume
(V2 ml)
| |
IBR
|
FBR
| ||||
1
|
10
| ||||
2
|
10
| ||||
3
|
10
| ||||
Calculation of strength of NaOH solution:
We know that, V1S1 = V2S2 Here, V1 = 10 ml
S2 =  S1 = N
S1 = N
V2 = ml
= S2 = ?
= N
Procedure for sample preparation and titration:
- 5 ml of milk of magnesia was taken in a conical flask.
- 25 ml of 1 N H2SO4 solution was added to it.
- After solution is complete, methyl red indicator 1-2 drops were added.
- The excess acid was titrated with 1 N NaOH which was previously kept on burette. When yellow colour appeared, addition of NaOH solution was stopped.
- The experiment was done for two times.
Data for standardization of Na2S2O3:
No. of observation
|
Volume of added H2SO4 solution
(V1 ml)
|
Volume of NaOH solution (ml)
|
Difference
(ml)
|
Mean volume
(V2 ml)
| |
IBR
|
FBR
| ||||
1
|
25
| ||||
2
|
25
| ||||
Calculation of percentage of Mg(OH)2:
= 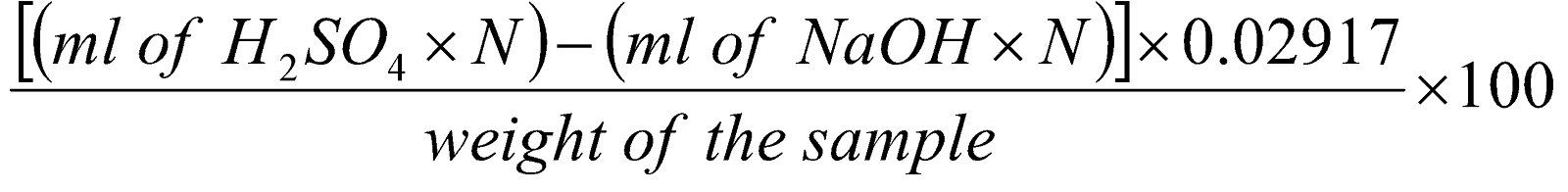
= 
= 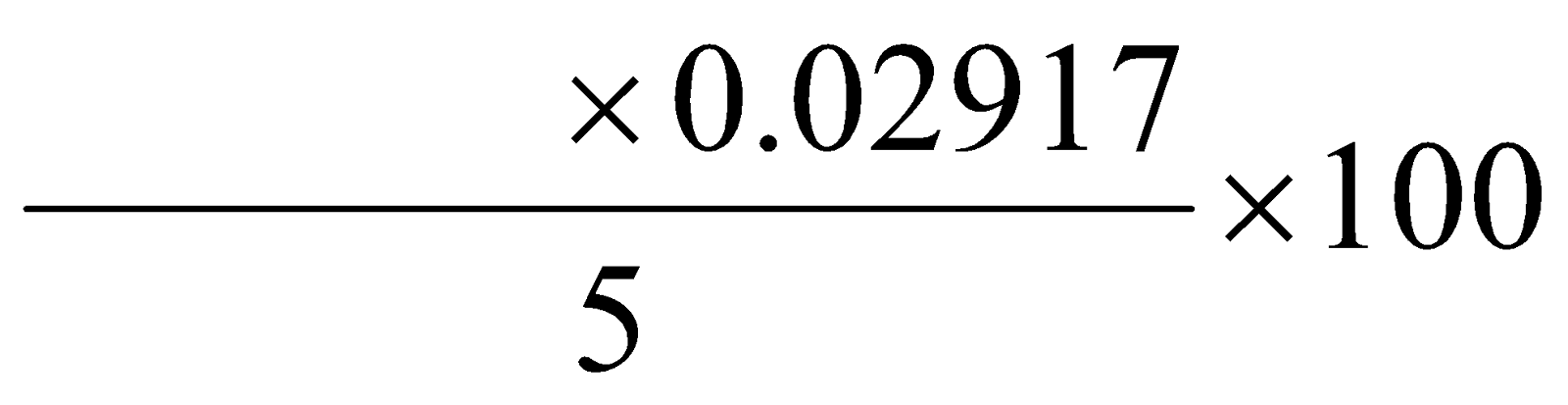
= %
Result:
The percentage of Mg(OH)2 present in sample was %.
Comments:
Experiment No. 03
|
Date: 08.02.12
|
Name of the experiment: Assay of ferrous fumerate from the supplied sample.
| |
Principle:
Assay of ferrous fumerate is done by carrying out an oxidation-reduction titration method using 0.1 N cerric ammonium sulfate [NH4(Ce) (SO4)2 . 2H2O] as a titrant and phenanthroline ferrous complex as an indicator.
At the end of the titration color of the titration medium changes into bluish green color. The supplied 0.1 N cerric ammonium sulfate solutions are previously standardized with Na2S2O3 solution, which in turn was standardized by K2Cr2O7 solution.
1 ml of cerric ammonium sulfate = 16.99 mg of ferrous fumerate.
Reagent and their preparation:
- 1 N 100 ml HCl solution: 8.5 ml 36.5% concentrated HCl (specific gravity 1.16) was taken in a volumetric flask and added water q. s. to 100ml.
- 0.1 N 250 ml Cerric ammonium sulfate solution: 16.5 gm of this reagent was taken into 150 ml of distilled water in a 250 ml of volumetric flask, 7.5 ml of concentrated H2SO4 was added and the flask was shaken for dissolving. Then the solution was heated at 37-40°C at water bath until a fresh color of the solution was obtained. Finally volume was adjusted up to the mark by adding distilled water.
- 0.1 N 500 ml Na2S2O3 solution: 12.4 gm of Na2S2O3 was taken into 500 ml volumetric flask. About 300 ml distilled water was added and shake well to dissolve. After that distilled water was added up to the mark.
- 0.1 N 100 ml K2Cr2O7 solution: 0.49 gm of K2Cr2O7 was taken in a 100 ml conical flask and mark up to with distilled water.
- Kl solution: 10 gm of KI was taken into 100 ml volumetric flask and water was added q. s. to 100 ml.
- Starch solution: 0.2 gm of starch was taken into 100 ml of water and heated up to complete the solution.
- Phenanthroline complex: 0.7 ml of ferrous sulfate was taken into a 100 ml volumetric flask and 1.5 gm of phenanthroline was added and water q. s. to 100 ml was added.
Standardization of 0.1 N Na2S2O3 solution by 0.1 N K2Cr2O7 solution:
- 10 ml of K2Cr2O7 solution was taken in a conical flask. 2 gm of NaHCO3 and 3 gm of KI was added to it and finally 5 ml conc. HCl was added.
- The conical flask was covered with a watch glass and kept for 5 minutes in a dark place.
- The watch glass was washed down with distilled water and the solution was diluted with about 50 ml of distilled water.
- The titration was performed by using 0.1 N Na2S2O3 solution which was previously kept on burette. When light yellow color appeared, the addition of thiosulfate solution was stopped and few drops of starch solution were added. It was again titrated with thiosulfate solution until blue color disappeared.
- The same experiment was done for three times.
Data for standardization of Na2S2O3:
No. of observation
|
Volume of K2Cr2O7 (V1 ml)
|
Volume of Na2S2O3 solution (ml)
|
Difference
(ml)
|
Mean volume
(V2 ml)
| |
IBR
|
FBR
| ||||
1
|
10
| ||||
2
|
10
| ||||
3
|
10
| ||||
Calculation of strength of Na2S2O3 solution:
We know that, V1S1 = V2S2 Here, V1 = 10 ml
S2 =  S1 = N
S1 = N
V2 = ml
= S2 = ?
= N
Standardization of Cerric ammonium sulfate by Na2S2O3 solution:
- 10 ml of cerric ammonium sulfate was taken in a 250ml conical flask and 15 ml KI solution was added.
- The titration was performed by using 0.1 N Na2S2O3 solution which was previously kept in burette. When light yellow color was appeared, addition of thiosulfate was stopped and 1ml of starch solution was added as an indicator. It was again titrated with this thiosulfate solution until blue color disappeared.
- The experiment was repeated for three times.
Data for standardization of Cerric ammonium sulfate:
No. of observation
|
Volume of cerric ammonium sulfate (V2 ml)
|
Volume of Na2S2O3 solution (ml)
|
Difference
(ml)
|
Mean volume
(V1 ml)
| |
IBR
|
FBR
| ||||
1
|
10
| ||||
2
|
10
| ||||
3
|
10
| ||||
Calculation of strength of cerric ammonium sulfate:
We know that, V1S1 = V2S2 Here, V1 = 10 ml
S2 =  S1 = N
S1 = N
V2 = ml
= S2 = ?
= N
Assay of ferrous fumerate in supplied sample by standard cerric ammonium sulfate:
Procedure:
- The supplied sample (i.e. average of 10 ferrous fumerate) was taken into a 50 ml volumetric flask.
- 10 ml of 1 N HCl was added to it and then boiled until the solid substance dissolves completely in a water bath.
- After cooling the volume was adjusted by adding distilled water.
- 25ml of resulting solution was transferred into a 250 ml conical flask and 3-4 drops of phenanthroline complex was added and titrated against 0.1 N cerric ammonium sulfate until bluish green color was appeared.
- The experiment was repeated for two times.
Table for final titration:
No. of observation
|
Volume of supplied solution
(ml)
|
Volume of cerric ammonium sulfate (ml)
|
Difference
(ml)
|
Mean
(ml)
| |
IBR
|
FBR
| ||||
1
|
25
| ||||
2
|
25
| ||||
Calculation:
1 ml of cerric ammonium sulfate = 16.99 mg of ferrous fumerate
- of ” ” ” =
” ”
= mg of ferrous fumerate
Now, 25 ml solution contains = mg of ferrous fumerate
∴ 50 ” ” ” = ” ” ” ”
= (P) mg of ferrous fumerate
∴ Potency = 
=
= %
Result:
The potency of ferrous fumerate was %.
Comments:
No comments:
Post a Comment