Laboratory Manual
Course: 310
Subject: Practical - Pharmacology-II

Course: 310
Subject: Practical - Pharmacology-II
Prepared By
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Md. Imran Nur Manik
M.Pharm
Departmnt of Pharmacy
Uniersity of Rajshahi
Rajshahi-6205, Bangladesh
Available at
Essential Pharma documents
www.pharmacydocs.blogspot.com
Essential Pharma documents
www.pharmacydocs.blogspot.com
INDEX
Sl. No.
|
Date
|
Name of the experiment
|
Page No.
|
01
|
Estimation of blood glucose level from the supplied sample.
|
2-5
| |
02
|
Determination of blood level of aspirin.
|
6-11
|
Experiment Number: 01
|
Date:12.09.2011
|
Name of The Experiment: Estimation of blood glucose level from the supplied sample.
| |
Introduction:
The blood sugar concentration or blood glucose level is the amount of glucose (sugar) present in the blood of a human or animal. A body's homeostatic mechanism, when operating normally, restores the blood sugar level to a narrow range of about 4.4 to 6.1 mmol/L (82 to 110 mg/dL). Controversially it varies from 80 to 120 mg/dL. Glucose levels are usually lowest in the morning, before the first meal of the day (termed "the fasting level"), and rise after meals for an hour or two by a few milliMolar.
Blood sugar levels outside the normal range may be an indicator of a medical condition. A persistently high level is referred to as hyperglycemia; low levels are referred to as hypoglycemia. A temporarily elevated blood sugar level may also result from severe stress, such as trauma, stroke, myocardial infarction, surgery, or illness. Intake of alcohol causes an initial surge in blood sugar, and later tends to cause levels to fall. Also, certain drugs can increase or decrease glucose levels.
Glucose is the primary source of energy for the body's cells, and blood lipids (in the form of fats and oils) are primarily a compact energy store. Glucose is transported from the intestines or liver to body cells via the bloodstream, and is made available for cell absorption via the hormone insulin, produced by the body primarily in the pancreas.
Units
The international standard way of measuring blood glucose levels are in terms of a molar concentration, measured in mmol/L (millimoles per litre; or millimolar, abbreviated mM). In the United States, mass concentration is measured in mg/dL (milligrams per decilitre).
Since the molecular weight of glucose C6H12O6 is about 180 g/mol, for the measurement of glucose, the difference between the two scales is a factor of 18, so that 1 mmol/L of glucose is equivalent to 18 mg/dL.
Principle:
The glucose of the supplied sample is determined based on the reaction of glucose with Fehling solution(A & B) to form glucoronic acid.
The color of the reaction solution is adjusted with arsenic-molybdate to form a color complex is measured by a spectrophotometer at 520 nm wavelength. The concentration of the glucose can be determined from a standard curve that is obtained by plotting the standard glucose concentration against absorbance.
Reagents:
- Fehling solution (A and B)
- 10% Na-tungstate
- Arsenic molybdate
- Heparin
- Glucose
Apparatus:
- Test tube
- Volumetric flask
- Measuring cylinder
- Graduate pipette
- Spectrophotometer
- Centrifuge machine
- Water bath
Preparation of Stock Solution:
- gm glucose was taken in 100ml volumetric flask and volume was adjusted tor 100ml mark by the addition of distilled water.
Concentration of stock Solution =0.1 gm/ml
=100mg/ml
=1mg/ml
=1000µg/ml
Preparation of 20, 40, 60, 80,100µg/ml standard solution:
1. Five volumetric flask ware washed, and marked as 2,4,6,8,&10 respectively.
2. Then 2,4,6,8,&10 ml of stock solution was taken in the corresponding volumetric flask so that they contain 20,40,60,80&100 µg/ml glucose respectively.
3. Finally the volume was adjusted to the 100 ml mark with distilled water.
Preparation of sample solution:
- Certain amount of glucose was dissolved in water and it was administered orally to an individual.
- After half an hour of ingestion, 3 ml of blood sample was withdrawn into a test tube containing 1 ml Na-oxalate.
- 0.5 ml blood, 0.5 ml Na-tungstate, 0.5 ml 2/3 (N) H2SO4 and 8.5 ml distilled water taken in a centrifuge tube and it was centrifuge for 5 minutes at about 4,500 rpm.
- 0.5 ml of supernatant serum was taken in a test tube. Then 0.5 ml Fehling solution was added and it is mixed well.
Procedure:
- Seven test tubes was taken and labeled as Blank, 2,4,6,8, 10 & Sample respectively.
- 0.5 ml of standard solution was taken separately from each of the volumetric flask by 1 ml pipette into corresponding labeled test tube and 0.5ml Fehling solution (A & B Mixed in 25:1 ratio) was added and mixed well.
- The Blank, Sample and five standard solution containing test tube was heated in a water bath for about 30 minutes. Then they ware cooled under tap water.
- Then Arsenic Moybdate reagent was added in each of the seven test tube with sufficient shaking and kept for some time to allow the reaction to occur completely.
- After that 8.5 ml of distilled water was added in each of the seven test tube with sufficient shaking.
6. Then the absorbance of the individual final solution prepared above, was taken in the spectrophotometer at 520 nm wavelength.
7. Finally a standard calibration curve for Glucose was obtained by plotting the absorbance (reading of the spectrophotometer) against the respective concentration.
8. At last the concentration of glucose in the blood sample was determined by calculating from the standard curve.
Data:
Data for the absorbance individual final solution (Blank was adjusted to zero):
Specimens concentration (µg/ml)
|
Absorbance
|
Concentration of glucose in the blood sample(µg/ml)
|
20
| ||
40
| ||
60
| ||
80
| ||
100
| ||
Blood Sample
|
Calculation:
The concentration of glucose in the blood sample determined; was calculated as follows.
Dilution factor = 20
Actual concentration of glucose = µg/ml
=  µg/dl
µg/dl
=
Result:
From the standard calibration curve it was found that the concentration of glucose in the blood sample
Was µg/dl
Comments: The blood sugar level of the individual was range.
Experiment Number: 02
|
Date:12.09.2011
|
Name of The Experiment: Determination of Blood Level of Aspirin .
| |
Introduction
Aspirin also known as acetylsalicylic acid (abbreviated ASA), is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. Salicylic acid, the main metabolite of aspirin, is an integral part of human and animal metabolism.
Principle:
Blood level of salicylic acid can be determined by chemical and spectrophotometric method. The serum is reacted with ferric mercuric reagent, precipitated plasma protein and simultaneously form a color complex with salicylic acid to give color. Developed color can be determined by spectrophotometric method.
Chemistry:
Salicylates are ester of salicylic acid with in possess analgesic, antipyretic and anti-inflammatory action.
Formula: C9H8O4 ; Mol. Mass : 180.157 g/mol
Suppression of prostaglandins and thromboxanes
Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (PTGS) enzyme required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the PTGS enzyme. This makes aspirin different from other NSAIDs (such as diclofenac and ibuprofen), which are reversible inhibitors.
Low-dose, long-term aspirin use irreversibly blocks the formation of thromboxane A2 in platelets, producing an inhibitory effect on platelet aggregation. This antithrombotic property makes aspirin useful for reducing the incidence of heart attacks. 
Pharmacokinetics:
Salicylic acid is a weak acid, and very little of it is ionized in the stomach after oral administration. Acetylsalicylic acid is poorly soluble in the acidic conditions of the stomach, which can delay absorption of high doses for eight to 24 hours. The increased pH and larger surface area of the small intestine causes aspirin to be absorbed rapidly there, which in turn allows more of the salicylate to dissolve. Owing to the issue of solubility, however, aspirin is absorbed much more slowly during overdose, and plasma concentrations can continue to rise for up to 24 hours after ingestion.
About 50–80% of salicylate in the blood is bound by protein, while the rest remains in the active, ionized state; protein binding is concentration-dependent. Saturation of binding sites leads to more free salicylate and increased toxicity. The volume of distribution is 0.1–0.2 l/kg. Acidosis increases the volume of distribution because of enhancement of tissue penetration of salicylates.
As much as 80% of therapeutic doses of salicylic acid is metabolized in the liver. Conjugation with glycine forms salicyluric acid, and with glucuronic acid it forms salicyl acyl and phenolic glucuronide. These metabolic pathways have only a limited capacity. Small amounts of salicylic acid are also hydroxylated to gentisic acid. With large salicylate doses, the kinetics switch from first order to zero order, as metabolic pathways become saturated and renal excretion becomes increasingly important.
Salicylates are excreted mainly by the kidneys as salicyluric acid (75%), free salicylic acid (10%), salicylic phenol (10%) and acyl glucuronides (5%), gentisic acid (< 1%) and 2,3-dihydroxybenzoic acid. When small doses (less than 250 mg in an adult) are ingested, all pathways proceed by first-order kinetics, with an elimination half-life of about 2.0 to 4.5 hours.[130][131] When higher doses of salicylate are ingested (more than 4 g), the half-life becomes much longer (15–30 hours), because the biotransformation pathways concerned with the formation of salicyluric acid and salicyl phenolic glucuronide become saturated. Renal excretion of salicylic acid becomes increasingly important as the metabolic pathways become saturated, because it is extremely sensitive to changes in urinary pH. There is a 10- to 20-fold increase in renal clearance when urine pH is increased from 5 to 8. The use of urinary alkalinization exploits this particular aspect of salicylate elimination.
Cardiovascular applications:
Aspirin is used to inhibit platelet aggregation. Low doses are used prophylactically to
1) reduce the risk of recurring transient ischemic attacks (TIAs) and stroke or death in those who have had single or multiple episodes of TIA or stroke;
2) reduce the risk of death in those having an acute myocardial infarction;
3) reduce the risk of recurrent nonfatal myocardial infarction and/or death in patients with previous myocardial infarction or unstable angina pectoris;
4) reduce the risk of myocardial infarction and sudden death in patients with chronic stable angina pectoris;
5) reduce the cardiovascular risk in patients undergoing certain revascularization procedures For long-term myocardial infarction prophylaxis, the dose is 81 to 162 mg/day; for those with RA or osteoarthritis, the initial dose is 3 grams/day; for stroke prophylaxis, the dose is 50 to 325 mg/day; in a patient having an acute mycardial infarction, the dose is 162 to 325 mg of nonenteric coated aspirin chewed and swallowed immediately.
Indication:
- Headache vi. Arthritis
- Rheumatic fever vii. Cholera
- Acute diarrhoea viii. Infertility
- Toothache ix. Threatened abortion
- Myalgia x. Prevention of heart attacks and strokes
Adverse effects:
- Urticaria vi. Anjioneuratic edema
- GIT disturbance vii. Rashes
- Shock viii. Bleeding tendency
- Asthma ix. Renal irritation
- Fever x. Iron deficiency anemia
Contraindication:
- Hypersensitivity of aspirin
- Patient with severe peptic ulcer
- Severe hepatic dysfunction
- Patient with anti-coagulant therapy
- Patient with clotting disorder
- Patient with vitamin-K deficiency
- During pregnancy.
Dose:
Normal dose: 300 mg thrice daily.
In rheumatoid arthritis: 4-6 gm/day.
Aspirin overdose can be acute or chronic. In acute poisoning, a single large dose is taken; in chronic poisoning, higher than normal doses are taken over a period of time. Acute overdose has a mortality rate of 2%. Chronic overdose is more commonly lethal, with a mortality rate of 25%; chronic overdose may be especially severe in children.
Interactions
acetazolamide and ammonium chloride have been known to enhance the intoxicating effect of salicyclates, and alcohol also increases the gastrointestinal bleeding associated with these types of drugs.
Aspirin displaces a number of drugs from protein binding sites in the blood, including the antidiabetic drugs tolbutamide and chlorpropamide, the immunosuppressant methotrexate, phenytoin, probenecid, valproic acid (as well as interfering with beta oxidation, an important part of valproate metabolism) and any nonsteroidal anti-inflammatory drug.
Corticosteroids may also reduce the concentration of aspirin. Ibuprofen can negate the antiplatelet effect of aspirin used for cardioprotection and stroke prevention. The pharmacological activity of spironolactone may be reduced by taking aspirin, and aspirin is known to compete with Penicillin G for renal tubular secretion. Aspirin may also inhibit the absorption of vitamin C.
Reagents:
- Spectrophotometer mercuric chloride
- Ferric nitrate [Fe(NO3)2. 9H2O]
- 1N HCl
- Heparin / Na-oxalate
- Acetyl salicylates
Apparatus:
- Spectrophotometer iv. Test tube
- Volumetric flask v. Measuring cylinder
- Centrifuge machine vi. Graduate pipette
Procedure
Preparation of sample solution:
- 300 mg of soluble aspirin was administered orally to an individual.
- After one hour of ingestion, 5 ml of blood sample was withdrawn into a test tube containing 2 ml of Na-oxalate.
- The blood sample was centrifuge at maximum speed of 4,000 rpm.
- 1 ml supernatant serum was taken in another test tube. 5 ml ferric mercuric reagent was added and mixed well.
- The mixture was again centrifuged at a maximum speed for 5 minutes for complete precipitation of the protein by forming complex with the reagent.
- The supernatant was taken as sample.
Preparation of bulk and stock solution:
- 7 test tubes were labeled as 10, 20, 40, 60, 80 and 100 μg/ml and one for blank solution.
- 50 mg of aspirin was dissolved in maximum of distilled water and the volume was made up to 500 ml by adding sufficient amount of distilled water.
- 10 ml of solution was taken from it in a 100 ml volumetric flask and the volume was made up to the mark with distilled water for 10 μg/ml.
- Similarly 20 ml for 20 μg/ml and 40, 60, 80 and μg/ml are respectively for 40, 60, 80 and 100 ml was taken in individual volumetric flask and the volume was made up to the mark by adding distilled water. There were standard solutions for different concentration.
- For blank solution, 1 ml distilled water and 5 ml ferric mercuric chloride reagent was taken in a test tube.
- 1 ml solution from every standard solution was taken in the respective test tubes that were heated in a water bath for two minutes and were cooled under running water.
- 5 ml of ferric mercuric reagent added to each of these test tubes & mixed well. These heated again in water bath for 3 minutes & cooled under running water.
- The absorbance was measured for 10, 20, 40, 60, 80,100 μg/ml and blank solution of 540 nm wavelengths in spectrophotometer.
- Finally, a standard six-point calibration curve for acetyl salicylic acid was obtained by plotting absorbance versus concentration.
Calculation:
500 ml contains = 50 mg of aspirin
1 ml contains = 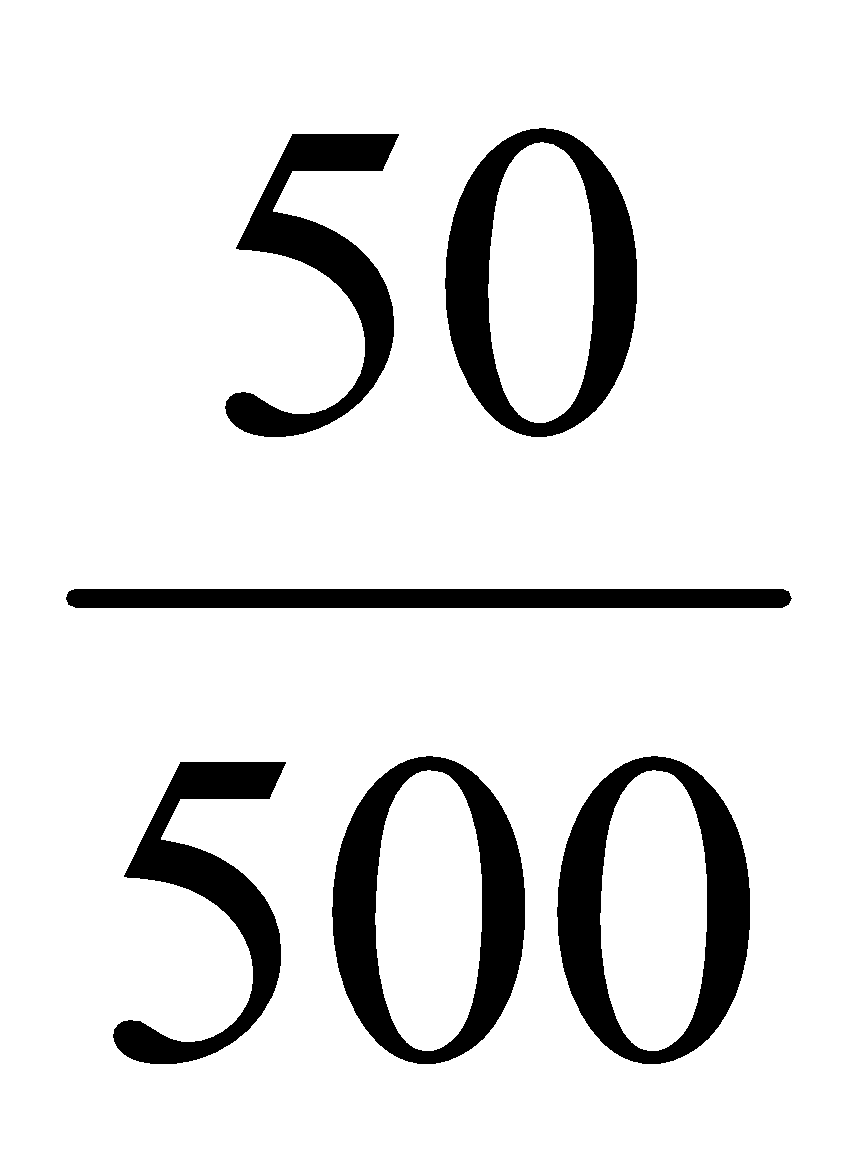 mg of aspirin
mg of aspirin
= 0.1 mg of aspirin
For 10 μg/ml:
1 ml contain = 0.1 mg of aspirin
10 ml contain = 0.1 × 10 mg of aspirin = 1 mg of aspirin
Then, 100 ml contains = 1 mg of aspirin
1 ml contains = 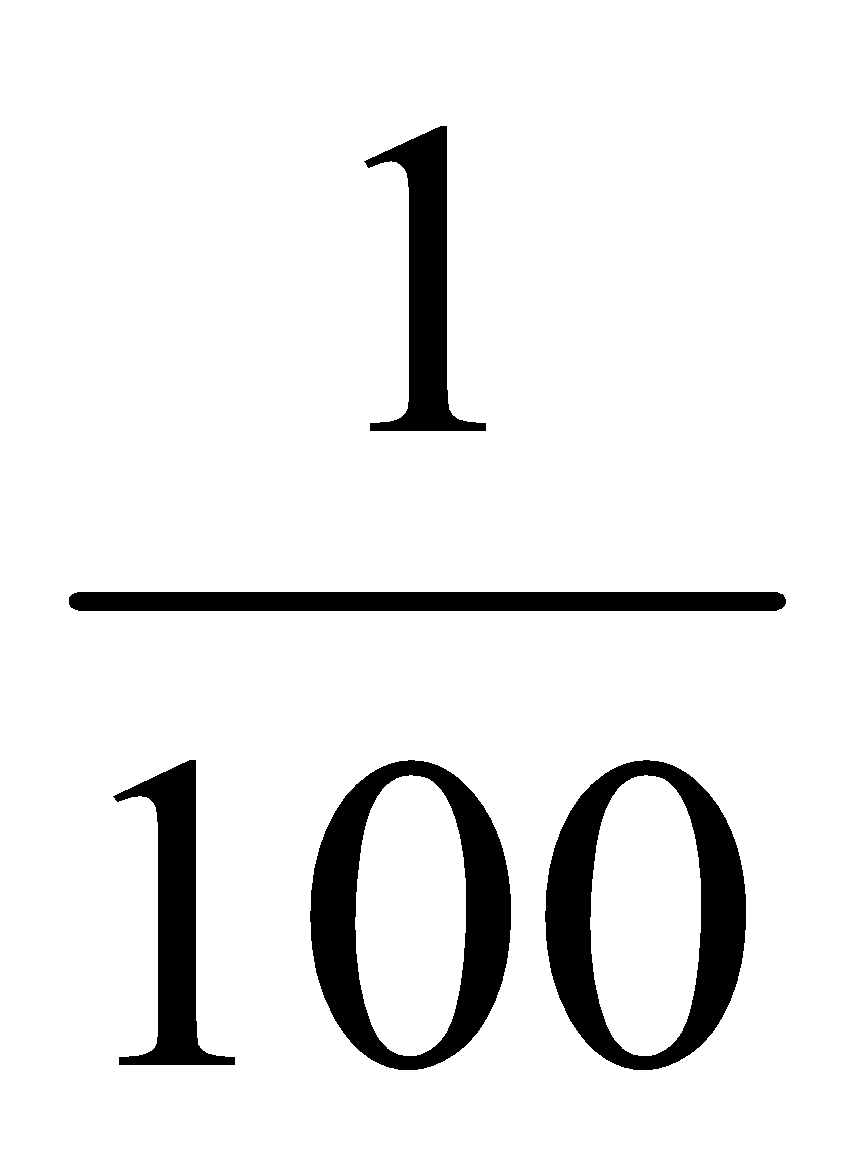 mg of aspirin
mg of aspirin
= 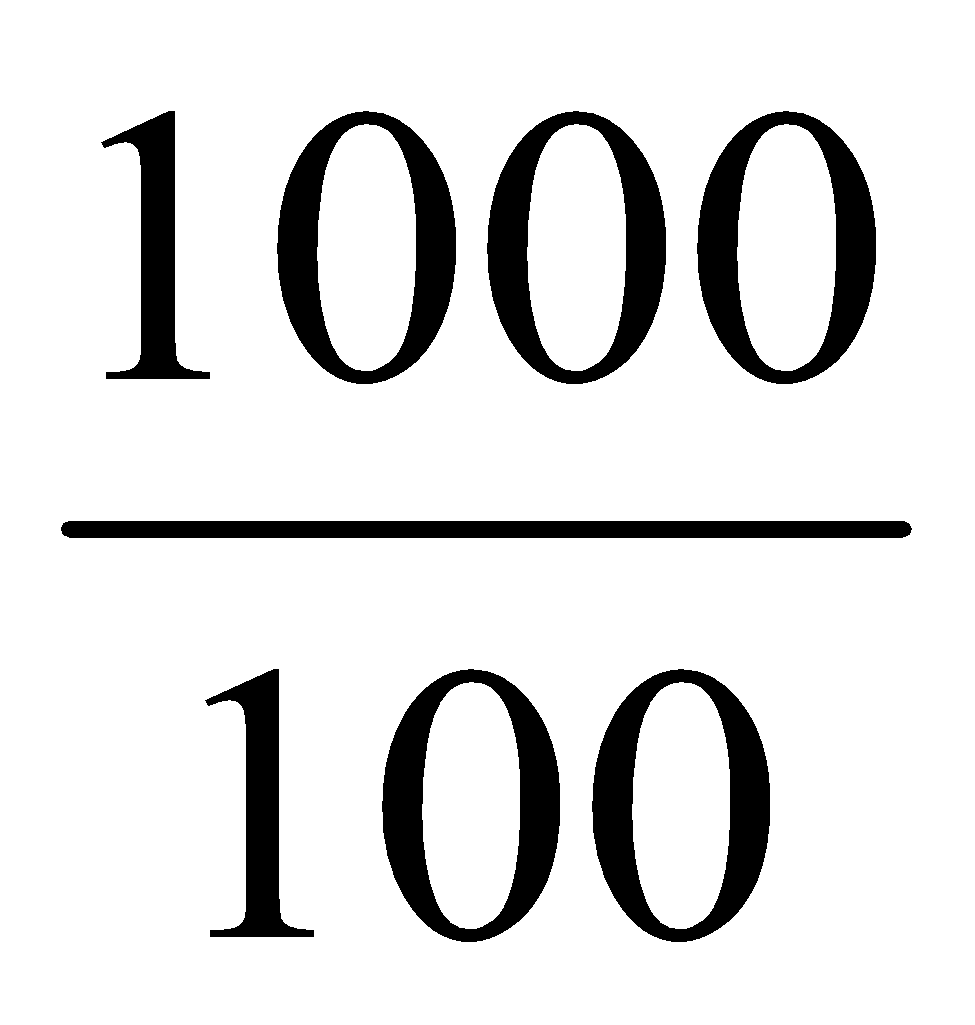 μg/ml = 10 μg of aspirin
μg/ml = 10 μg of aspirin
For 20 μg/ml:
1 ml contains = 0.1 mg of aspirin
20 ml contains = 20 × 0.1 mg of aspirin = 2 mg of aspirin
Then, 100 ml contain = 2 mg or 2000 μg of aspirin
1 ml contain = 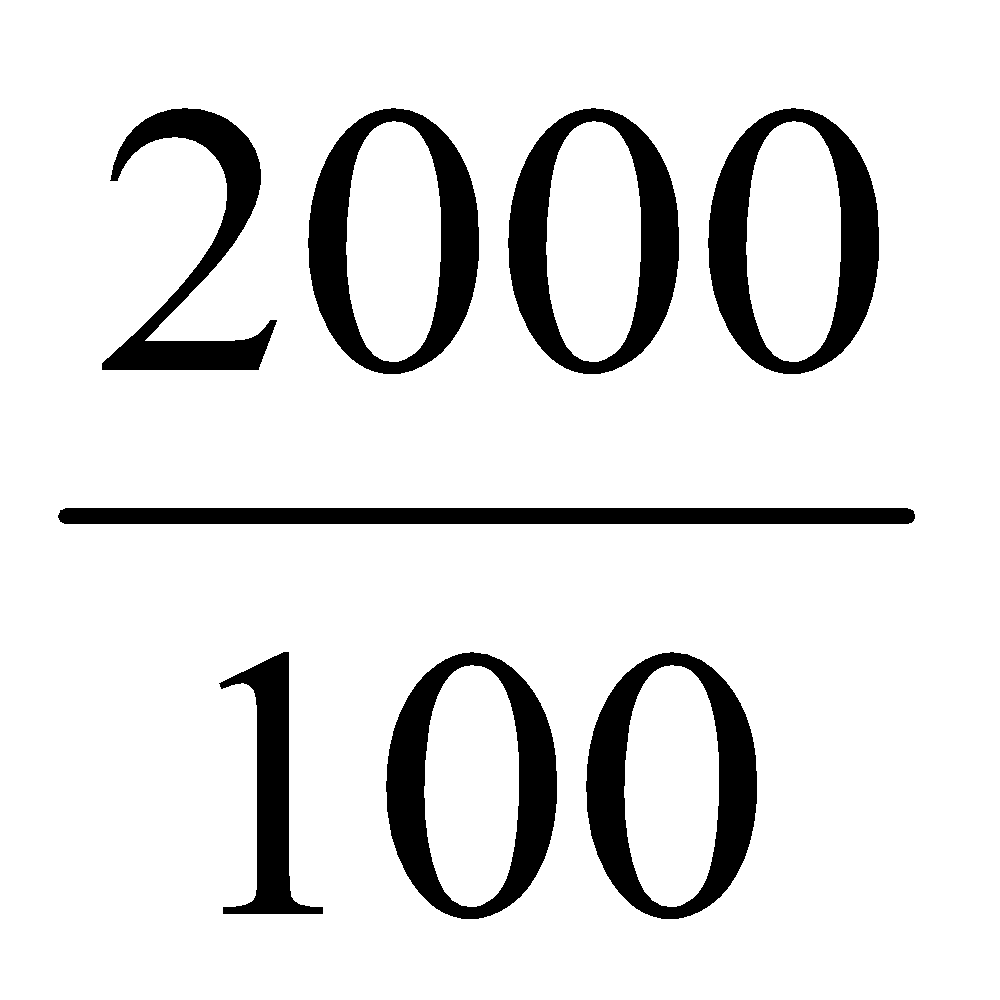 mg of aspirin
mg of aspirin
= 20 μg/ml
Similarly, 40 ml, 60ml, 80 ml and 100 ml taken for 40 μg/ml, 60 μg/ml, 80 μg/ml and 100 μg/ml respectively.
Data: Data for the absorbance of blank solution was:
Specimen concentration (μg/ml)
|
Absorbance (nm)
|
Result of glucose concentration in blood(μg/ml)
|
Result:
After experiment from the calibration curve it was found that, the concentration of salicylic acid in blood sample was = μg/ml.
Comment:
We know that the blood level of aspirin more than that of 500 μg/ml Shows toxicity.
Our experiment level of aspirin in blood is μg/ml.
Therefore, it is in therapeutic level & gives analgesic, antipyretic & anti-platelet effect.
No comments:
Post a Comment