3235: Fluorometry
Fluorometry
Terminologies
Luminescence: Luminescence is the phenomenon of a chemical species to absorb radiation of UV or visible region and emit a radiation of longer
wavelength. Loss of energy and concomitant transition of molecules from excited states to ground states with emission of radiation is called luminesce
nce.
What happens here is, energy excites the molecules (more specifically electrons of the molecules). When the molecules return to the normal state,
they emit radiation–light.
Luminescence can be divided into two types depending on the lifespan of the excited state –
Fluorescence
Phosphorescence
Fluorescence: Fluorescence is defined as the emission of radiation by a chemical species during its transition from an excited singlet state to the
ground (singlet) state. The extent of fluorescence can be measured by fluorometry.
At room temperature, in normal condition, molecules will be at ground state and bonding electrons will spin in opposite directions. Energy
level is lowest. Excitation requires energy which must be supplied in the form of UV or visible light. Following light absorption, a
chromophore is excited to some higher vibrational energy level of S1 or S2. Then, due to vibrational relaxation, the molecules will descend
to the lowest vibrational energy level of the excited state. This process is radiationless but energy is lost in other forms.
From the lowest vibrational level of excited state, molecules will return to the ground state by emission of radiation. Since, little energy is
lost during vibrational relaxation, the radiation emitted has lower energy than the radiation absorbed. Hence, in fluorescence emitted
radiation has longer wavelength.
In fluorescent molecules, luminescence stops within 10-8 to 10-4 seconds. It is important to remember that, the molecule will undergo
vibrational relaxation to the lowest vibrational energy level before returning to the ground state to give fluorescence. So
The phenomenon of radiation emission during transition from the lowest vibrational energy level of the excited singlet state to the ground
state is called fluorescence.
Phosphorescence: Phosphorescence is defined as the emission of radiation by a chemical species during its transition from the excited triplet state
to the singlet ground state.
The triplet state of a molecule has a lower energy than its associated singlet state so that transitions back to the ground state are
accompanied with the emission of light of lower energy than from the singlet state. Therefore, we would typically expect phosphorescence
to occur at longer wavelengths than fluorescence. Phosphorescence is often characterized by an afterglow because of the long life of the triplet
state,10-4-10 seconds.
An important feature of phosphorescence is afterglow. Light is emitted from phosphorescent molecules after radiation energy source is
removed. This is because the luminescence continues for 10-4 seconds to 10 seconds as the triplet state has greater longevity.
In phosphorescence, similar to the fluorescence, vibrational relaxation must occur.
Singlet state: Singlet state is the state in which all of the electrons are paired and in each pair the two electrons spin about their own axis in
opposite directions.
Excited singlet state: When two electrons of the singlet state are goes to the excited state it is called excited singlet state. In excited singlet state
electrons remain as in exciting position.
Triplet state: Triplet state is a state lying at an energy level intermediate between ground and excited state and characterized by an impairing of two
electrons.
In contrast to the singlet state, there is a spin reversal involving one electron of the pair and the pair of two electrons spins about their axis in the
same direction. The life time of the molecule in the triplet state in 10-4 to 10 seconds.
Vibrational energy level:
Even at ground state a molecule is always vibrating. Therefore the energy at ground state is not a single discreet value rather a set of discreet values.
In another words there are different energy levels in the molecule duo to its vibration.
Whether the molecule is in ground state or excited state, the molecule contains many energy levels which are called vibrational energy levels.
There may be many Occupied Molecular Orbitals (bonding orbitals) and many Unoccupied Molecular Orbitals
(antibonding orbitals-electrons transit to these during excitation). Now, when a molecule is in ground state, the electrons will not transit to
the antibonding orbitals. But they may transfer from one bonding orbital to another. This causes vibration of the molecules.
Again, when a molecule is in excited state, one electron from each pair of bonding electrons will transit to the antibonding orbitals. When
they are in these antibonding orbital, they may transfer from one antibonding orbital to another.
Vibrational relaxation: Vibrational relaxation is the transition of molecule from any of the vibrational energy levels to the lowest vibrational energy
level of the excitatory state.

Energy lost in this process is thought to be via thermal process probably lost to solvent molecules.
Resonance fluorescence: Resonance fluorescence is the phenomenon where the molecule absorbs and emits equal amount of energy. Practically
resonance fluorescence doesn’t occur or occur rarely as vibrational relaxation occurs.
Internal conversion: The phenomenon of excited molecule to return to the ground state by losing energy by means other than photo radiation is
termed internal conversion.
Intersystem crossing: The transfer of a molecule present in the lowest vibrational energy level of the excited singlet state to an excited triplet state
is called intersystem crossing.
Differences between fluorescence and phosphorescence:
Property
|
Fluorescence
|
Phosphorescence
|
Transition
|
Molecule transits from excited singlet state to ground state.
|
Molecule transits from excited triplet state to ground state.
|
Lifespan
|
Fluorescence is continued for only 10-8 to 10-4 seconds.
|
Phosphorescence continues for 10-4 seconds to 10 seconds.
|
Afterglow
|
Not present.
|
Occurs and luminescence slowly fades.
|
Analytical application
|
Yes.
|
No.
|
Quantum efficiency: Quantum efficiency is defined as the ratio of number of light quanta emitted and the number of light quanta absorbed.

Its significance is that, it is an indicator of how fluorescent a molecule is. If Q is near 1, the molecule is highly fluorescent molecule and if Q is near 0,
the molecule is a very low fluorescent molecule.
Fluorometry
The method of analysing a sample by measuring its fluorescence i.e. intensity and composition of light emitted by it, is called fluorometry.
Fluorescence spectroscopy aka fluorometry or spectrofluorometry is an analytical technique for identifying and characterizing minute amounts of a
fluorescent substance by excitation of the substance with a beam of ultraviolet light and detection & measurement of the characteristic wavelength of
the fluorescent light emitted.
It is a spectrochemical method. These terms are explained with the illustration below –
Theory of fluorometry
When energy is applied to certain molecules in the form of UV or visible electromagnetic radiation, the molecules temporally transit to an excited
singlet state where the excited electron is in paired condition with the ground electron. In the excited state, the molecules lose energy in radiationless
manner to descend to the lowest vibrational energy level of the excited state. The excited state lasts only 10-8 to 10-4 seconds and then the excited
molecule will return to ground state by losing energy through emitting radiation. This is termed fluorescence and the emitted radiation is of longer
wavelength.
By measuring the emitted wavelength we can determine the presence and amount of a compound in a sample.
1. When a molecule absorbs radiant energy it is got promoted from the ground state to the excited state and gets distributed in the various vibrational
energy levels mostly to the excited singlet state.
2. Radiationless vibrational relaxation to the lowest vibrational energy level of the excited singlet state: Molecules initially undergo a more rapid
process, a radiationless loss of vibrational energy and so quickly falls to the lowest vibrational energy level of the excited state, known as vibrational
relaxation.
3. Radiationless internal conversion (from excited singlet state to ground state followed by vibrational relaxation): From the lowest vibrational energy
level of the excited singlet state, a molecule can return to the ground state by photoemission or by radiationless process followed by vibrational
relaxation.
When an excited molecule undergo a radiationless loss of vibrational energy, sufficient to drop to the ground state then it is termed internal conversion.
4. Fluorescence (Followed by vibrational relaxation):The radiation emitted in the transition of a molecule from a singlet excited state to a singlet
ground state is called fluorescence.
The radiation emitted as fluorescence is of lower energy and therefore of longer wavelength than that originally absorbed.
5. Intersystem crossing (From excited singlet state to excited triplet state): Molecule in the lowest vibrational energy level of the excited singlet state
converts to a triplet state (the state lying at an energy level intermediate between ground state and excited).This process is called intersystem crossing. Here
molecules do not losses energy.
6. Vibrational relaxation (to the lowest vibrational energy level of the excited triplet state): Once intersystem crossing has occurred, a molecule so
quickly falls to the lowest vibrational energy level of the excited triplet state by vibrational relaxation. The lifetime of molecule in the triplet state is
10-4 to 10 seconds (Longer than corresponding singlet state).
7. Radiationless internal conversion from excited triplet state to ground state followed by vibrational relaxation: Here energy is released in the form of
heat radiation.
8. Phosphorescence (Followed by vibrational relaxation). The emission of radiation emitted in the transition of a molecule from a triplet excited state
to a singlet ground state is called Phosphorescence. It is characterized by afterglow because of the long life of the triplet state.
Relationship between fluorescence and chemical structure
Definite correlations between chemical structure and fluorescence can’t be made. But there is influence of structural features on the fluorescence of
organic compounds.
Degree of conjugation: Conjugation is necessary for fluorescence. This is because mobile π electrons are responsible for UV-Vis absorption
characteristics of compounds. Thus cyclohexane (saturated, no π electron) is not fluorescent, benzene is weakly fluorescent and anthracene is highly
fluorescent.
Delocalization of electron: In mono-substituted benzene derivatives following rules can apply –
Electron donating groups i.e. ortho-para directors increase fluorescence intensity as they increase electron density (increase electron
delocalization). E.g. fluoro, amino, hydroxy, methoxy group etc.
Electron withdrawing groups i.e. meta directors decrease fluorescence intensity as they decrease electron density (causes π electron localization)
. E.g. carboxyl, nitro, sulfonyl, aldehyde group etc.
Exception: Nitrile group even though meta directing, increases fluorescence intensity.
Compound
|
Fluorescence compare to benzene
|
Higher
|
Lower
|
Benzaldehyde
|
|
|
Chlorobenzene
|
|
|
Aniline
|
|
|
Nitrobenzene
|
|
|
Benzoic acid
|
|
|
Phenol
|
|
|
It was postulated that electrons of the CN group interacted with the π electrons of the benzene ring to result in a distribution that favoured fluorescence.
In di-substituted benzene derivatives fluorescence is unpredictable. For example, aniline is a fluorescent compound. When a meta directing group
e.g. sulfamoyl group is added (then the compound is sulfanilamide) fluorescent intensity increases 5 times.
Although it may be expected that substitution of the fluorescent compound, aniline, with a meta-directing group —SO2NH2 would result in a compound which would fluoresce
to a lesser degree than aniline.But Sulfanilamide, however, was found to be five times as fluorescent as aniline.
Rigidity and planarity: The higher the rigidity the greater is the fluorescence intensity. This is because, rigidity and planarity will prevent vibration
and free rotation of aromatic rings hence less energy is dissipated in radiationless manner.
(Fluorescein , is highly fluorescent, while phenolphthalein is nonfluorescent. The oxygen bridge in fluorescein imparts rigidity and planarity that is not present in phenolphthalein. the vibrational
energy is greater.)
cis-trans isomerism: It also affects fluorescence intensity. Generally trans isomers have greater fluorescence than corresponding cis isomers. This is
due to non-planar character of cis isomers.
Heterocylic compounds:
A double-bonded nitrogen (=N—) generally decrease the fluorescence intensity but  generally increase the fluorescence intensity.
generally increase the fluorescence intensity.
Ionization: Many compounds show fluorescence at ionized state. But this is dependent upon pH of the solution.
Complexation: Complexation increases rigidity and minimizes internal vibration hence fluorescence intensity is increased.
e.g. Tetracycline has a weak native fluorescence but complexes of the antibiotic with Ca2+ and a barbiturate fluorescence quiet intensely.
Tetracycline complexes
(non fluorescent) (fluorescent)
Chemical conversion
Acid treatment-
Hydrocortisone is not fluorescent itself but they from strongly fluorescence compound in concentrated H2SO4 in the prescence of ethanol.
Hydrocortisone strongly fluorescent compound.
Oxidation
By oxidation and hydroxylation epinephrine forms strongly fluorescing compound.
Epinephrine Highly fluorescent compound.
Thiamine is not itself fluorescent ,but it`s oxidation product thiochrome is fluorescent.
Thiamine Thiochorme
Instrumentation
In fluorometry the intensity of radiation emitted as fluorescence related to the concentration of the fluorescing species is measured.
The instrumentation is for measuring the intensity of fluorescence as a function of the wavelength of the radiation.
The chief components are:
Light source
Filter (Primary Filter) /monochromater
Sample holder
|
A emission filter (Secondary Filter) / emission monochromater
Detector
Recorder and Amplifier
|
Radiation source:
To produce exciting light, radiation source is required. The radiation source must be intense and stable. Mercury arc and Xenon arc lamp are
commonly used.
The emission of a mercury lamp is concentrated in several very intense bands. Among those having a wavelength of 254-365 nm are of a great value as excitation
radiation is evenly distributed over a wide range of wavelengths.
Excitation filter:
It filters the source light and isolates the band of exciting light that is to be passed to the sample holder. If the instrument uses coarse monochromator
then the instrument is called fluorometer. If grating or prism monochromator is used then the instrument is called spectrofluorometer,
spectrophotofluorometer or florescence spectrometers. Usually glass filters are used.
Sample holder:
Glass cells are used for most analysis. If measurement is to be under 320nm wavelength then quartz cells are used.
Emission filter:
It selects the band of fluorescence which is to be detected. It is usually placed at right angle (90º) to the beam of exciting (transmitting) light but other
arrangements are possible.
Detector:
A photomultiplier and phototube is used to detect the fluorescent light and amplify it.
(The detector is placed at a right angle to the direction of travel of beam of exciting light.)
Recorder:
The output of the detector is connected to a meter, a digital display or a recorder. Recorder gives the intensity of radiation in terms of electrical signal
produced by the detector.
Factors influencing intensity of fluorescence
Concentration of fluorescing species
Presence of other solutes or impurities
pH of the sample solution
Stability of the sample compound
Solvent effects
Temperature
Concentration of fluorescing species:
Fluorescence intensity (F) can be described as follows –
The exponential form of the Beer’s law (Beer-Lambert law) is
By putting the value of I from equation (2) in equation (1) we get –
Thus we can see that the relationship between fluorescence intensity and concentration is quite complex. But from the above equation,
When c increases,  value decreases and thus F value increases. So we can say that fluorescence will increase with increase of concentration of
fluorescing species.
value decreases and thus F value increases. So we can say that fluorescence will increase with increase of concentration of
fluorescing species.
Concentration reversal:
Concentration reversal is the phenomenon where the fluorescence intensity decreases as a result of increase in concentration.
For some chemical species, if the supplied energy is fixed but the concentration is increased gradually then at one point the fluorescence will decrease.
This is because; the supplied energy fails to excite all the molecules present in the solution at a time. So when the excited molecules emit energy
(this is the fluorescence), the previously unexcited molecules will absorb that energy. The emitted energy measured is less i.e. the fluorescence
intensity is less.
For example the supplied energy can excite 20 molecules. If 25 molecules are present in the solution then, the energy will excite 20 molecules and
5 molecules will remain unexcited. When the excited molecules emit energy the unexcited molecules will absorb that energy.
However, if the sample is concentrated, sufficient light absorption might occur so that the portion sensed by the detector is only weakly irradiated. This results
in the phenomenon of concentration reversal.
Presence of other solutes/impurities:
Fluorescent impurities:
The sample solution may contain components other than the sample which is fluorescent. These interfere with accurate measurement of fluorescence
of the sample compound. Thus precautions such as use of pure solvent and chemical reagents, cleanliness in all operations should be taken.
Inner-filter effect:
It is the reduction in the fluorescence intensity due to presence of non-fluorescent solutes which retard penetration of light to or from fluorescent
molecules.
Non-fluorescent molecules either prevent incident light from reaching the fluorescent molecules (absorption retardation) or prevent emitted light
detection.
Remedy: the non-fluorescent absorber must be eliminated or be maintained constant from sample to sample and a standard curve must be used which was determined at that concentration of
absorber. Or, the wavelength of excitation or emission radiation to minimize this effect.
Chemical quenching:
Chemical quenching is the decrease of fluorescence intensity due to presence of any chemical in the sample solution. i.e.
It is a chemical process where a chemical species reduce fluorescence intensity.
The chemical responsible for quenching is called quencher.
There are two types of quenching –
Collisional quenching: When the quencher absorbs the energy emitted by the excited fluorescent molecules, it is called collisional quenching.
Halide ions e.g. iodide, chloride ions cause it.
A molecule of “quencher" interacts with an excited molecule of the potentially fluorescing substance. Interaction results in the dissipation of excitation energy not by fluorescence but by transfer
of energy to the quenching molecule.
Static quenching: When the quencher absorbs the incident light in place of fluorescent molecules, it is called static quenching. Xanthines
(caffeine) and purines cause it for vitamin B12.
pH of the sample solution:
Intensity of fluorescence is dependent upon the pH of the solution. This is due to two reasons –
Degree of ionization: In weak electrolytes pH affect the degree of ionization. Now, ionized and unionized species may have different
fluorescence intensity. Again, it is possible that ionized species is fluorescent but the unionized species is not and vice versa. Thus pH may
affect the fluorescence intensity of a compound.
Exemplary, 2-naphthol ( ) shows fluorescence in both ionized and unionized forms. But ionized form give fluorescence peak at 429µm
whereas unionized form gives fluorescence peak at 359µm. So, if we measure fluorescence at 429µm (actually a filter is used to omit radiation below
415µm) then only the fluorescence of ionized species can be detected; this is detected at pH 8.5 and above [Degree of ionization is detectable at pH
equal to
) shows fluorescence in both ionized and unionized forms. But ionized form give fluorescence peak at 429µm
whereas unionized form gives fluorescence peak at 359µm. So, if we measure fluorescence at 429µm (actually a filter is used to omit radiation below
415µm) then only the fluorescence of ionized species can be detected; this is detected at pH 8.5 and above [Degree of ionization is detectable at pH
equal to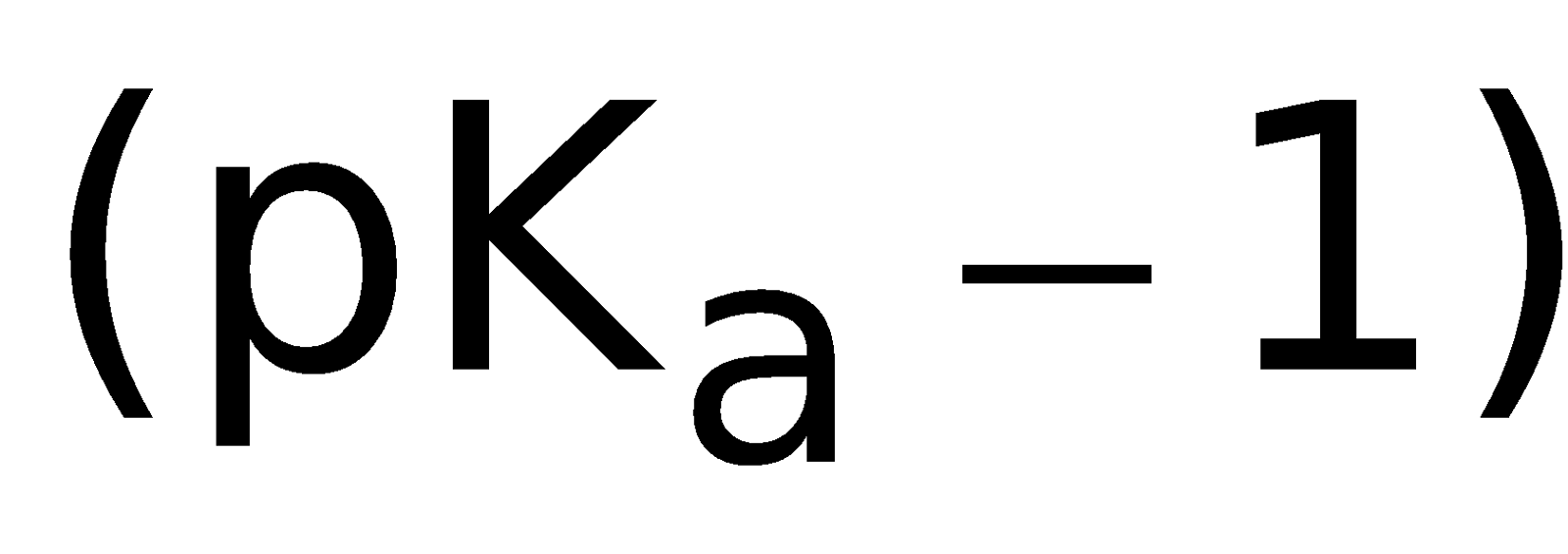 ].
].
Excited-state dissociation: Sometimes, it is possible that a compound has different acid strength in ground state and in excited state. So, if the
excited state acid strength is greater, then the compound will dissociate more easily when in excited state. Then, difference in the fluorescence
intensities of ionized and unionized species will cause change in fluorescence.
Exemplary, when fluorescence is measured at 429µm (a filter is used to omit radiation of below 415µm) fluorescence is detected in the pH range of
2-8.5. But ground state 2-naphthol undergoes detectable ionization at pH 8.5 and above. Excited state 2-naphthol underwent ionization in the pH
range 2-8.5, which is why we get fluorescence in that range.
Stability of the sample compound (Degradation of Sample)
If the compound being analysed is unstable in the experimental condition then fluorescence intensity will change. Causes of instability may be due
to –
Solvolytic degradation
Auto oxidation
Photo decomposition
Chemical degradation
Photodecomposition can be reduced by decreasing the intensity of the incident light. Also all measurements should be completed as quickly as
possible to avoid above problems.
Solvent effect:
Temperature:
Temperature reduces the fluorescence intensity. This is because of –
Increased internal conversion
Reduction in vibrational relaxation
In general, a 1⁰C rise in temperature results in a decrease of fluorescence intensity by 1%.
Comparison of Fluorometry with spectrophotometry
Sensitivity: Fluorometry is significantly more sensitive as an analytical tool than spectrophotometry.
The points included are.
In fuorometry the intensity of fluoresced light is measured directly by a fluorometer.
In spectrophotometry the intensity of light transmitted by a sample is measured and compared to that transmitted by a blank.
The directly measured intensity can be amplified more readily and accurately in fuorometry than the intensity difference measured in
spectrophotometry.
In case of spectrophotometry the lower limit of detectability is determined by the smallest concentration that will yield a detectable intensity
difference between sample and blank. Here small errors made in measuring the difference between the two intensities result in large errors in
calculated concentration.
The lowest limit of conc. that can be detected with accuracy is established by the molar absorptivity in spectrophotometry (12mg/dl). The
lower limit of conc. in fluorometry is established by characteristics of the instrument and not usually by characteristics of the following species.
Fluorescence measurements can offers sensitivity increases of 103-104 over absorbance measurements.
Specificity:
Fluorometric assay can offer a degree of specificity that might not be attainable with a corresponding spectrophotometric technique. The equations
relating fluorescence intensity to concentration hold for any region of the spectrum.
Experimental Variables:
There are large no of experimental variable that must be controlled in fluorometric methods of analysis than in corresponding spectrophotometric
methods.
The temperature and the intensity of incident light must be maintained reasonably constant in a fluorometric method, but not in a
spectrophotometric procedure.
Extraneous solutes can markedly affect the intensity of fluorescence by quenching affects, but it does not happen in spectrophotometry.
The influence of pH on fluorescence can be more complex than on absorbance and might necessitate closer control of pH in fluorometric
procedures than in spectrophotometric assays.
Difference between Absorption spectroscopy and Fluoroscence spectroscopy
Features
|
Absorption spectroscopy
|
Fluoroscence spectroscopy
|
Theoretical consideration
|
Measurement of amount of light absorbed.
|
Measurement of intensity of fluorescence.
|
Wavelength of light used
|
Which gives maximum absorption.
|
Which gives maximum fluorescence.
|
Instruments
|
Determines only the absorption of light.
|
Determines absorption of light as well as emission of radiation.
|
Light source
|
Tungsten, H2-discharge lamp.
|
Mercury arc lamp, Xenon arc lamp.
|
Cell used
|
Silica cell.
|
Glass and metal cells.
|
Detector
|
Phototube or photo multiplier is used to detect the radiation absorbed
|
Emission filter is used to separate the emitted light from the transmitted light.
|
Concentration
|
Concentration depends on the molar absorptivity.
|
Concentration depends on the characteristics of the instrument.
|
Electrical transition
|
Applicable for both π→π* & n→π* transition.
|
Not applicable for the compound containing n→π* transition.
|
Experimental variables
temperature & Extraneous solution
|
Not so restricted.
|
Highly restricted.
|
Sensitivity and selectivity
|
Less sensitive and less specific.
|
More sensitive and highly specific.
|
Applications of fluorometry
Application in Chemistry:
Fluorometry is used in chemistry for –
Determination of metal ions: Complexes of metals ions may give strong fluorescence which is utilized for this purpose.
Separation and identification: In many cases, after separation, chemicals are identified using fluorometry. e.g. aminocrine.
Application in Biopharmaceutics:
Measurement of drug in blood, urine and other body fluids.
Study of the rate and mechanism of drug absorption, metabolism and excretion.
Selection of toxic compounds.
Pharmaceutical applications:
Fluorometry is used for quantitative analysis of –
Hormones: Adrenaline, aldosterone, testosterone
Alkaloids:
Opioids: Morphine, codeine etc.
Rauwolfia alkaloids: Reserpine
Others: Atropine, emetine etc.
Vitamin: Riboflavin and thiamine are indicated for fluorometric assay by USP and BP.
Antibiotics: tetracycline, sulfonamide etc.
Cardiac glycosides: Such as digoxin, digitoxin, etc.
Fluorometry is also used for qualitative analysis of these drugs.
Fluerometry is widely used in the analysis of drugs in systems (physiological systems) other than dosage forms. The sensitivity of the method of
analysis is applied for a large number of pharmacological, biochemical, toxicological, pharmacokinetic (ADME) & biopharmaceutical studies for
the analysis of amount of drugs in biological fluids and tissues.
Advantages of fluorometry
1. Sensitivity: In case of Fluorescence, detectability to parts per billion or even parts per trillion is common for most analytes. This extraordinary
sensitivity allows the reliable detection of fluorescent materials (chlorophyll, aromatic hydrocarbons, etc.) using small sample sizes. Also, field studies
can be performed in open waters without sample treatment. Fluorometers achieve 1,000 to 500,000 times better limits of detection as compared to
spectrophotometers.
2. Specificity: Spectrophotometers merely measure absorbed light and as many materials absorb light, it becomes difficult to isolate the targeted
analyte in a complex matrix. Fluorometers are highly specific and less susceptible to interferences because fewer materials absorb and also emit light
(fluoresce).
And, if non-target compounds do absorb and emit light, it is rare that they will emit the same wavelength of light as target compounds.
3. Wide Concentration Range: Fluorescence output is linear to sample concentration over a very broad range. Fluorometry can be used over three
to six decades of concentration without sample dilution or modification of the sample cell.
6. Simplicity and Speed: Fluorometry is a relatively simple analytical technique. Fluorometry's sensitivity and specificity reduce or eliminate the
sample preparation procedures often required to concentrate analytes or remove interferences from samples prior to analysis. This reduction in or
elimination of sample preparation time not only simplifies, but also expedites the analysis.
7 Low Cost: Reagent and instrumentation costs are low when compared to many other analytical techniques, such as gas chromatography and
HPLC.
Reagent costs are low because, due to the high sensitivity of fluorometers, fewer reagents can be used. And, small laboratory filter fluorometers can now be
purchased for less than $3,000 USD.
Limitations of Fluorometry
1. Molecules should be fluorescent to measure by the fluorescence spectroscopy.
2. All kind of materials on substances cannot be detected by it.
References
Lakowicz, J.R. 1983. Principles of Fluorescence Spectroscopy, Plenum Press, New York.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York. 3 Id., p. 7.
Dr. Richard Thompson. 1998. University of Maryland, Department of Biochemistry and Molecular Biology, School of Medicine.
G. K. Turner, "Measurement of Light From Chemical or Biochemical Reactions," in Bioluminescence and Chemiluminescence: Instruments and Applications,
Vol. I, K. Van Dyke, Ed. (CRC Press, Boca Raton, FL, 1985), pp. 45-47.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York, pp. 51-57.
Lakowicz, J.R. 1983. Principles of Fluorescence Spectroscopy, Plenum Press, New York, chap. 2.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York, pp. 67-69.
Lakowicz, J.R. 1983. Principles of Fluorescence Spectroscopy, Plenum Press, New York, pp. 23-26.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York, pp. 57-58.
Stotlar, S. C. 1997. The Photonics Design and Applications Handbook, 43rd Edition, Laurin Publishing Co., Inc., Pittsfield, MA, p. 119.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York, p. 63.
Dr. Richard Thompson. 1998. University of Maryland, Department of Biochemistry and Molecular Biology, School of Medicine.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York, p. 30.
Dr. Richard Thompson. 1998. University of Maryland, Department of Biochemistry and Molecular Biology, School of Medicine.
Iain Johnson, Product Manager, and Ian Clements, Technical Assistant Specialist (May 1998 communication from Molecular Probes, Eugene, Oregon).
Fluorometric Facts: A Practical Guide to Flow Measurement, Turner Designs (1990), pp. 14-15.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York, p. 172.
Fluorometric Facts: A Practical Guide to Flow Measurement, Turner Designs (1990), p. 21.
Guilbault, G.G. 1990. Practical Fluorescence, Second Edition, Marcel Dekker, Inc., New York., p. 28.
Teitz Textbook of Clinical Chemistry and Molecular diagnosis (5th Edition)
Dr.B.K.Sharma, Instrumental methods of chemical analysis.
Gurdeep R Chatwal, Instrumental methods of chemical analysis
http://en.wikipedia.org/wiki/Fluorescence
http://images.google.co.in/imghp?oe=UTF-8&hl=en&tab=wi&q=fluorescence
-





