1101: Chemical Bond
Chemical Bond
A chemical bond is defined as the attractive force that holds two or more atoms together in a molecule or an ion.
Why do atoms combine? (Cause of chemical bonding)
By a close study of atoms and molecules it has been found that atoms combine chemically for the following reasons:
1. Net attractive force between atoms:
Atoms consist of strongly positive nucleus and negative electrons. When two atoms come closer to combine with each other to form a bond between them, the attractive and repulsive forces begin to operate between them. The attractive forces are between the electrons of one atom and the nucleus of other atom while the repulsive forces are between the electrons or the nuclei of the two atoms.
When the two atoms approach each other these forces counteract each other. The net result of these forces may be either attraction or repulsion between the atoms.
If the attractive forces become dominant over the repulsive forces, the net result is the attraction between the atoms and hence they combine together to form a chemical bond between them.
On the other hand if the repulsive forces become dominant over the attractive forces, the atoms do not combine and hence no chemical bond is established between them
Attractive Force > Repulsive Force → Formation of Bond
For example in case of hydrogen atoms the net result is attraction and two hydrogen atoms combine together to form H2 molecule. On the other hand in case of He atom the net result is repulsion and hence two He atoms do not combine together to form He2 molecule.
2. Octet rule or rule of eight: (Electronic Theory of valency or octet theory of valency)
The atoms of noble gases do not normally react with other atoms to form compounds. It is assumed that the outermost shell configuration of the atoms of noble gases is a stable configuration of 8 electrons which is known as octet. The two electrons in case of He is also stable as octet which is known as doublet.
Noble gas
|
Atomic Number
|
Electronic Configuration
|
He
|
2
|
2
|
Ne
|
10
|
2,8
|
Ar
|
18
|
2,8,8
|
Kr
|
36
|
2,8,18,8
|
Xe
|
54
|
2,8,18,18,8
|
Rn
|
86
|
2,8,18,32,18,8
|
The tendency of the atoms to have 8 electrons in their outermost shell is known as octet rule or rule of eight. Since helium atom has two electrons, this rule is called doublet rule or rule of two.
Octet rule was given in the form of a theory which is known as octet theory of valency or electronic theory of valency which states that: “In the formation of a chemical bond atoms interact with each other by losing, gaining or sharing of electron to acquire a stable outer shell of eight electrons (stable noble gas configuration)”.
Valence is the number of bonds formed by an atom in a molecule.
|
The atoms possessing less than 4 electrons generally tends to lose them while those having more than 4 electrons in the outer most shell tends to gain the electrons during the chemical combination or bond formation to attain stable configuration of the nearest inert gas.
3. Lowering of energy of combining atoms:
When two atoms combine together to form a bond there is an overall decrease in the potential energy of the combining atoms, which has greater stability.
Potential energy curve:
The curve shown below represents the variation of potential energy between the nuclei of the two atoms A and B which are approaching closer to each other to form a bond between them.
The trend of the curve from right to left should be observed.
When two atoms A and B are far away, say at an infinite distance from each other the attraction between them is zero. Hence there is no possibility of formation of bond between them. This situation has been represented by X.
As the two atoms brought closer to each other i.e. as the distance between the atoms is decreased, the attractive forces between the nucleus and the electrons become more dominant than the repulsive forces, operating between the electrons of the two atoms and hence energy of the system goes on decreasing as shown by the downward trend of the curve. This decrease of energy continues till a certain minimum value shown by Y in the curve is obtained.
Now if the atoms are brought still closer the repulsive forces between the two nuclei at such small internuclear distance becomes dominant and hence the energy of the system starts increasing as shown by the upward trend of the curve.
How Do Atoms Combine?
The process by which the atoms of the elements rearrange their outer-most shell electrons to get eight-electron outer-most shell configuration which is a stable configuration takes place by the formation of chemical bond.
Types of Chemical Bond
In general there are two main classes of bonds (or linkages) which hold the atoms together in a molecule. These two main classes are subdivided into small groups.
| |||||
↓
| |||||
|
| ||||
↓
|
↓
| ||||
1. Ionic or Electrovalent Bond
|
1. Hydrogen Bond
| ||||
2. Covalent Bond
|
2. Van Deer Waals Interaction
| ||||
3. Co-ordinate or Deviate Bond (Coordinate covalent bond)
| |||||
4. Metallic Bond
| |||||
1 Ionic or Electrovalent Bond (Bonding by Transference of Electrons)
The chemical bond formed between two atoms by the transfer of one or more valence electrons from one atom to the other is called ionic bond. This bond is also called electrovalent or polar bond.
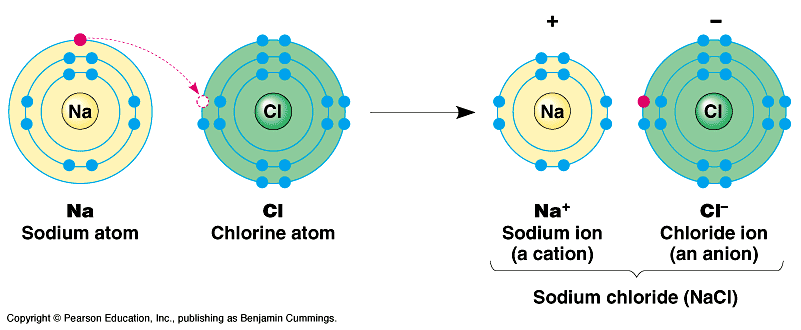
Illustration of the formation ionic bond:
Let us consider two atoms A & B; atom A has one electron in its valence-shell while atom B has seven electrons. Thus A has one electron in excess and B has one electron short than the stable octet. Therefore A transfers an electron to B and converts into positive ion (cation), A+. On the other hand, B accepts an electron from A and converts into negative ion (anion), B–. In this transaction both the atoms acquire a stable electronic configuration of their nearest inert element. The resulting cation and anion are held together by electrostatic force of attraction which is called ionic bond or electrovalent bond.
Examples of Ionic Compounds: NaCl, MgO, CaF2, Al2O3.
Factors favoring the formation of ionic bond
1) Number of Valence electron:
The atom which is converted into cation should possess 1, 2 or 3 valence electrons while the atom which is converted into anion should have 5, 6 or 7 valence electrons. The element of group IA, IIA and IIIA satisfy this condition for the cation and those of group VA, VIA and VIIA satisfy this condition for anion.
2) The ionization energy of the metal atom should be low. (Ionization energy is the minimum amount of energy required to remove the most loosely bound electron from a neutral isolated gaseous atom. If an atom has low ionisation energy, it will be easy for it to lose the electron and hence get converted into a cation. Hence low ionization energy of the metal will favour the formation of cation. e.g. Elements of groups Let IA and IIA have a strong tendency to turn into cations.)
3) Electron affinity of the nonmetal should be high. (Electron affinity is the amount of energy released when an electron is added to a neutral isolated gaseous atom. Since the release of energy is the sign of stability of a system, the atoms with high electron affinity will form the anions quite easily. e.g. The higher elements of groups VA and VIA and those of group VILA, have a strong tendency to turn into anions.)
4) The lattice energy of the ionic compound formed should high:
The energy released when one gram mole a crystal is formed from its gaseous atoms is called the lattice energy of the crystal. Thus:
A+(g) + B–(g) A+B–(crystal) + Energy released (Lattice energy)
1 mole 1 mole 1 mole
Higher the value of the lattice energy of a crystal, the greater is the ease of its formation i.e. greater will be the stability of the ionic crystal.
5) Electronegativity difference of the two atoms forming the ionic bond should be high:
Atoms will form an ionic bond, if they have greatly different electronegativities. In fact a difference of 2 or more is essential for the formation of ionic bond. For example, since the electronegativity difference between Na and Cl is 2.1 (Na=0.9, Cl=3.0) Na and Cl will form an ionic bond in NaCl molecule.
Electrovalency
Electrovalency of an element is its capacity to combine with an ionic compound.
Electrovalency of an element is equal to the number of electrons lost by atom of that element in forming a positive ions or gained by it in forming a negative ion, both having the noble gas configuration s2p6 configuration in their outermost shell.
(The elements which lose electrons show positive electrovalency while the elements which gain electrons show negative electrovalency For example in the formation of NaCl the electrovalency of Na is equal to +1 while that of Cl is equal to —1. An element which gains or loses one, two, three, four etc. electrons is called mono (or uni) valent, di (or bi) valent, trivalent, tetravalent etc. element.)
Example:
Na, Cl, F……… Monovalent elements
Mg, Ca, O …… Divalent elements
Al, B…………... Trivalent elements
C, Si…………… Tetravalent elements
Variable electrovalency
An element shows different combining capacity in different ionic compounds i.e. shows different value of electrovalency. This phenomenon is called variable electrovalency which is due to the following two reasons:
1) Unstable configuration of the core:
The outermost levels of the elements like Fe, Co, Ni, Cu etc contain only one or two electrons.
When these two electrons are lost, the remaining part which is called the core or kernel, is unstable hence more electron from the core can be further lost, giving variable electrovalency to the ions.
For example:
When these two electrons are lost, the remaining part which is called the core or kernel, is unstable hence more electron from the core can be further lost, giving variable electrovalency to the ions.
For example:
The variable electrovalency of iron is equal to +2 in ferrous compound and equal to +3 in ferric compound can be explained in as follows:
Fe (26) 1s22s2p63s2p6d64s2
Fe++(24) 1s22s2p63s2p6d6
3s2p6d6 configuration is unstable and hence loses one more electron and thus forms Fe+++ ion.
Fe+++(23) 1s22s2p63s2p6d5
The variable electrovalency of Cu equal to +1 (cuprous ion) and +2 (cupric ion) is easily understood from the electronic configurations given below.
Cu (29) 2, 8, 18, 1 or 2, 8, 3s2p6d10, 4s1
Cu+ (28) 8, 8, 18 or 2, 8, 3s2p6d10
Cu2+ (27) 2, 8, 17 or 2, 8, 3s2p6d9
2) Inert electron pair effect:
Some of the heavier elements of p-block of the long form of periodic table like those of group IIIA, IVA, VA and VIA show two oxidation state as shown below:
Group IIIA (G=3) → (+1,+3)
Group IVA (G=4) → (+2,+4),
Lower oxidation state is obtained when only np electrons are lost and the ns electron pair, due to extra stability, remains inert, i.e. it is not lost. Such a pair of ns electrons is called inert electron pair and the effect caused by this is called inert electron pair effect.
Properties of ionic compounds
1. Physical state: Ionic compounds are crystalline solids at room temperature.
(Ionic compounds consist of three dimensional solid aggregates of cations and anions which are arranged in a well-defined geometrical pattern. They are never liquids or gases under the ordinary conditions of temperature and pressure.)
2. Electrical conductivity: Ionic compounds do not conduct electricity when they are in the solid state. The ionic solids conduct electricity when they are water solution or in the molten state.
(Molten ionic compounds or their aqueous solutions conduct a current when placed in an electrolytic cell.)
(Molten ionic compounds or their aqueous solutions conduct a current when placed in an electrolytic cell.)
3. They are quite hard, have low volatility (have low vapour pressure) and high melting and boiling points.
(Since, very high amount of energy (in the form of heat) is required to separate the cations and anions from one another against the electrostatic forces of attraction.)
4. Solubility in polar and nonpolar solvent: Ionic solids are freely soluble in polar solvents like H2O, liquid ammonia etc.
(Because the electrostatic force of attraction holding the cation and anion together in the ionic solid is reduced by the high magnitude of the dielectric constant value of the polar solvent.
The solubility of ionic solids in a polar solvent like water can also be explained by saying that a water molecule is a dipole and hence the positive end of water dipole interacts with the negative ion of the ionic solids and the negative end of the dipole interacts with the positive ion of the same ionic crystal as shown in Fig. 7.6 in which the dissolution of NaCl ionic crystal in water has been shown.)
On the other hand ionic solids are insoluble or slightly soluble in nonpolar solvents (organic solvents) such as C6H6, CCl4 etc. 
(Such solvents, due to their low value of dielectric constant, do not allow the ions to move freely and interact with them to form the solvated ions.)
5. Crystal structure: Ionic solids do not exist as individual neutral independent molecules rather they exist as three dimensional solid aggregates which have definite geometric shape.
(In order to occupy minimum space the ions arrange themselves systematically in an alternating cation-anion pattern called crystal or lattice. Thus we see that ionic solids consist of three dimensional solid aggregates.)
6. Highly brittle: Ionic solids are highly brittle, i.e. if a little external force is applied on ionic crystals, they are easily broken. This property is called brittleness.
We know that ionic solids are composed of parallel layers which contain cations and anions in alternate positions so that the opposite ions in the various parallel layers lie over each other. When a little external force is applied on an ionic crystal one layer of ion slides a bit over other layer along a plane. As a result like ion come in front of each other and hence begin to repel each other. Consequently the application of a little external force brings about repulsion between the two layers and the ionic solid breaks.
Hence ionic solids are highly brittle.
Hence ionic solids are highly brittle.
7. High density: The electrostatic force of attraction existing between the cation and anion in an ionic crystal bring these ions very close to each other. This decreases the volume of crystal and as a consequence this ionic crystal has high density.
8. Stability: Ionic crystals are very stable compound
9. They do not exhibit isomerism: Ionic bond involving electrostatic lines of force between opposite ions is non-rigid and non-directional. The ionic compounds are, therefore, incapable of exhibiting stereoisomerism.
2 Covalent bond (Bonding by Mutual Sharing of Electrons)
The attractive force of the two nuclei for the shared pair of electrons holds the atoms together and gives rise to the formation of a bond which is called covalent bond or electron pair bond and the compounds containing covalent bonds are called covalent compounds.
Definition: The chemical bond between two atoms in which the electrons (in pair) are shared by both the participating atoms is called covalent bond. (Since a covalent bond between atoms results by the interaction of their electrons which become common to both the atoms, it is also called an atomic bond.)
Illustration of the formation of covalent bond:
Hydrogen atom has one valence electron and oxygen atom (2,6) has six valence electrons, and achieves the stable octet by sharing two electrons with two hydrogen atoms (one with each H atom. Thus hydrogen atoms acquire 2 electrons and oxygen acquires 8 electrons in their respective outermost shells and the shared electron pair contributes a covalent bond between hydrogen atoms and oxygen. Some Other Examples: H2, N2, O2 NH3 ,BF3, CH4 molecules
Types of Covalent Bond (Single, double and triple covalent bonds)
The covalent bond formed by the sharing of one, two or three electron pairs between the participating atoms is called single, double and triple covalent bond respectively.
Double and triple covalent bonds are called multiple covalent bonds.
Type
|
Representation
|
No. of Electron Pairs
|
Example
|
Single Covalent bond
|
Single Dash (–)
|
1 pair=(1×2)=2 electrons
|
H–H, Cl–Cl, H–Cl, H–O–H
|
Double Covalent bond
|
Double Dash (=)
|
2 pairs=(2×2)=4 electrons
|
O=O
|
Triple Covalent bond
|
Triple Dash (≡)
|
3 pairs=(3×2)=6 electrons
|
N≡N
|
Covalency
The valency of an element in a covalent compound is called its covalency. The covalency of an atom in a covalent compound is equal to the number of covalent bonds formed by one atom of it with the neighboring atom.
Variable covalency
The covalency of an element which has only s and p orbitals in its valence shell is equal to the total number of unpaired electrons in s or p orbitals. For example: Covaleny of H atom is 1 (H→1s1) and O atom is 2 (O→2s22px22py12pz1).
The element containing vacant d-orbitals show different values of covalency in different covalent compounds. For example: Phosphorus (3, 5), Sulphur (2, 4, 6).
Factors favoring the formation of covalent bond
(conditions for the formation of covalent compounds)
(conditions for the formation of covalent compounds)
1. High ionization energy: Atoms which have high value of ionization energy are incapable of losing electrons to form cations. Thus these elements cannot form ionic bonds. Rather, they, due to their high ionisation energy, can form covalent bonds between them.
2. Equal electron affinities: For covalent bonding the two atoms must have almost equal attraction for electrons. (In other words the combining atoms must have almost equal electron affinities.)
3. Number of valence electron: Each of the two atoms should have 5, 6 or 7 valence electrons
(H atom has only one electron) so that both the atoms achieve the octet by sharing 3, 2 or 1 electron pair. The nonmetals of VA, VIA and VIIA groups respectively satisfy this condition.
(H atom has only one electron) so that both the atoms achieve the octet by sharing 3, 2 or 1 electron pair. The nonmetals of VA, VIA and VIIA groups respectively satisfy this condition.
4. Equal electronegativity: Both atoms should have equal electronegativity so that the transfer of electron(s) from one atom to another may not take place. (i.e., ionic bond may not be formed)
5. High nuclear charge and small internuclear distance: High charge on the bonding nuclei and smaller internuclear distance favor the formation of covalent bond.
Properties of covalent compounds
1. Physical state: Most of the covalent compounds are present as gases or liquids of low boiling points under the normal condition of temperature and pressure due to weak forces.
(However, they may exist as soft solids only when their molecular weights are high; e.g. chlorine (mole. wt. = 71) is a gas, bromine (mol. wt. 160) is a liquid, while iodine (mol. wt. = 254) is a solid.)
2. Covalent compounds don't conduct electricity in water: Electricity is conducted in water from the movement of ions from one place to the other. These ions are the charge carriers which allow water to conduct electricity. Since there are no ions in a covalent compound, they don't conduct electricity in water.
Another Explanation: Covalent solids consisting of giant molecules are bad conductors of electricity, since they do not contain charged particles (i.e. ions) or electrons to carry the current. However the covalent solids having the layer lattices (e.g. graphite) are good conductors of electricity, since in such solids electrons can pass from one layer to the other and thus current can be carried. Certain covalent substances like HCl ionise in solution and their solutions conduct electricity.
3. Lower melting and boiling points: Except giant molecules other covalent solids have relatively lower melting and boiling points than the ionic solids. Low boiling points are due to the fact that the attractive forces between the covalent molecules are weak van der Waal’s forces.
4. Solubility in polar and nonpolar solvents: Covalent solids are insoluble in polar solvents like H2O but are readily soluble in nonpolar solvents like C6H6, CCl4 etc. Their solubility in nonpolar solvents is based on the principle ‘like dissolves like’.
(Covalent solids having the giant molecules are insoluble in all the solvents. This is because of the fact that these solids, due to their big size, are not able to interact with the solvent molecules. Some of the covalent compounds like alcohol; amines etc. are soluble in H2O due to hydrogen bonding.)
5. Crystal structure: Crystals of solid covalent compounds are of two types: 
Those in which every atom is bonded with other atoms by covalent bonds resulting in the formation of giant molecule. Example: Diamond, silicon carbide (SiC), aluminium nitride (AlN) etc.
Those which consist of separate layers. The covalent compounds containing separate layers are said to have layer lattice structure. Example of such compounds is: CdI2, CdCl2, BN, graphite etc.
6. Neither too hard nor too brittle: Covalent compound are neither hard nor brittle. Rather they are soft and waxy, since they usually consist of separate molecules.
7. Isomerism: Since covalent bonds are rigid and directional, they can give rise to different arrangement of atoms in space. So a single molecular formula of a covalent compound may represent a number of different compounds with different properties. Thus covalent compounds can show isomerism.
Comparison of the properties of ionic and covalent compounds
Property
|
Ionic Compounds
(Electrovalent compounds) |
Covalent Compounds
|
1. Physical State
|
They are crystalline solids at room temperature. They are never liquids or gases under the ordinary conditions of temperature and pressures
|
Most of them (covalent compounds) exist as gases or liquids. However, the covalent compounds having high molecular weight can exist as solids.
|
2. Crystal Structure
|
They consist of 3D solid aggregates (ionic solids)
|
The crystals of the covalent com pounds are of two types (a) those consisting of giant molecules (e.g. diamond etc.) and (b) Those consisting of separate layers (e.g. graphite, CdI2 etc.).
|
3. Hardness and brittle ness
|
These are hard and brittle. Since the cations and anions are held together by very strong electrostatic force of attraction.
|
Solid covalent compounds are soft and waxy, since they usually consist of separate molecules. They are much readily broken.
|
4. Nature of reactions
|
They undergo ionic reactions (in solution) which are fast and instantaneous.
|
They undergo molecular reactions
(in solution) which are slow. |
5. Melting and boiling points
|
They have usually high melting and boiling points, since very high amount of energy(in the form of heat) is required to separate the cations and anions against the forces holding them together in the ionic solids.
|
With the exception of giant molecules, other covalent solids have low melting and boiling points. This is because of the reason that the attractive forces between covalent molecules are weak van der Waal’s forces.
|
6. Solubility
|
They are freely soluble in polar solvents and insoluble in or slightly soluble in nonpolar solvents. Their solubility in polar solvents is due to the greater solvation energy then the lattice energy of ionic solid.
|
They are insoluble in polar solvents and readily soluble in non-polar solvents. Their solubility in nonpolar solvents is based on the principle” like dissolves like”
|
7. Electrical conductivity
|
These are bad conductors when they are solid. However they conduct electricity in fused state or in solution in which their ions are free to migrate.
|
Covalent solids consisting of giant molecules are poor conductors because they do not contain charged particles or electrons to carry the current
|
8. Nature of bonds
|
(a) Formed by the transfer of electrons from metal to a non-metal. (b) Consist of electrostatic force between cations and anions.(c) non rigid and non-directional
|
(a) Formed by the sharing of electrons pair (s) between non-metal atoms. (b) Consist of shared pair (s) or electrons between atoms.(c) rigid and directional
|
9. Isomerism
|
Rare
|
Frequent
|
Covalent compounds tend to be more flammable than ionic compounds.
The main reason is that, materials burn because they contain carbon and hydrogen atoms, which can react to form carbon dioxide and water when heated with oxygen gas (definition of combustion). Since carbon and hydrogen have very similar electro negativities, they are mostly found together in covalent compounds. As a result, more covalent compounds than ionic compounds are flammable.
There are a couple of exceptions to this rule. The first is, with covalent compounds containing neither carbon nor hydrogen do not have tendency to b urn and if they burn, they do so by mechanisms other than the classic combustion reaction. The other exceptions come with ionic compounds referred to as “organic salts”. These organic salts are ionic compounds in which the anion is basically a big covalent molecule containing carbon and hydrogen with just a very small ionic portion. As a result they burn as though technically they are ionic compound.
Failure of octet rule in covalent compounds
There are many covalent molecules in which the central atom which is covalently bonded with other atoms has electrons either less than eight (incomplete octet) or more than eight (expansion of octet) in its outermost shell.
A) Incomplete octet: For example, BeCl2, BF3, NO etc.
BeCl2 molecule: in this molecule has only four electrons in its outermost shell (two of its own and the other two from the two covalently bonded Cl atoms).
B) Expansion of octet: For example, PCl5, ICl3, SF6, IF7 etc.
PCl5 molecule: In PCl5 molecule the total number of electrons in the outermost shell of phosphorus atom is ten (five electrons of its own and five electrons gained by it in forming five covalent bonds with five chlorine atoms).
Explanation of the Failure of Octet Rule.
Sugden’s concept of single linkage: Sugden postulated that the central atom of the molecules like PCl5, maintains its octet and in doing so the central atom is linked with some of the combining atoms by singlet-electron bonds, called singlet linkages while with the remaining atoms it is linked by the normal two electron covalent bonds. The singlet linkage is a special type of bond which is formed by the one sided sharing of only one electron between the central atom and the combining atoms.
Sidgwick’s concept of maximum covalency: The maximum covalency of an element actually depends on the period in which the element is present.
Period
|
Maximum covalency
|
First period (n = 1)
|
2
|
Second period (n = 2)
|
4
|
Third and fourth periods (n = 3 & 4)
|
6
|
Higher periods (n > 4)
|
8
|
Covalent bonds having partial ionic character
1) Non polar covalent bond: A covalent bond between two similar atoms which have the same electronegativity is called a nonpolar covalent bond. For example: H2, F2 etc.
If the atoms are equally electronegative, both have the same tendency to attract the bonding pair of electrons, and so it will be found on average half way between the two atoms.
2) Polar covalent bond:
A covalent bond between two dissimilar atoms which have different electronegativity values is called a polar covalent bond. For example: H2O, NH3 etc.
B will attract the electron pair rather more than A does. That means that the B end of the bond has more than its fair share of electron density and so becomes slightly negative. At the same time, the A end becomes slightly positive. In the diagram, δ (read as "delta") means "slightly" - so δ+ means "slightly positive".
Percentage of ionic character in a polar covalent bond
Electronegativity difference (XA - XB)
|
% ionic character in
A-B bond
|
Nature of A-B bond and its representation
|
Examples of bonds
|
0
|
0
|
Purely covalent
|
N2, O2
|
0.1-0.8
|
0.5-15
|
Covalent
|
C-S, N-Cl
|
0.9-1.6
|
19-47
|
Polar covalent
|
H2O
|
1.7
|
50
|
50% ionic and 50% covalent
| |
> 1.7
|
> 50
|
Ionic
|
NaCl
|
Dipole: The molecules like HCl which have polar covalent bonds are called polar molecules.
A polar molecule which has positive and negative charge centers or electrical poles becomes dipolar and hence is called a dipole.
A polar molecule which has positive and negative charge centers or electrical poles becomes dipolar and hence is called a dipole.
Dipole moment: The product of the total amount of positive or negative charge and the distance between the positive and negative centers (bond length) is called dipole moment.
Larger the dipole moment of a molecule, greater will be its degree of polarity.
Electronegativity
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7.
Polarisation of ions
The ability of a cation to polarise a nearby anion is called its polarizing ability and the tendency of an anion to get polarized by a cation is called polarisability.
This phenomenon is called polarization of anion by cation.
This phenomenon is called polarization of anion by cation.
Greater the magnitude of polarizing power of a cation or polarisability of an anion, greater the amount of covalent character produced in the molecule.
Factors affecting the polarisability of an anion (Fajan’s rule)
1. Charge on cation or anion: Higher the positive charge on the cation, greater its polarizing power to polarise a given nearby anion. For example: Li+ < Be2+ < B3+ < C4+
Higher the negative charge on the anion, more strongly it will be polarized by a given cation.
C4- > N3- > O2- > F-
2. Size of the cation: Smaller the size of the cation, higher is its polarizing power to polarise a given nearby anion. Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
3. Size of the anion: Larger the size of the anion, more strongly it will be polarized by a given cation. F- < Cl- < Br- < I-
4. Electronic configuration of the cation: Cation with 18 electron valence shell configuration (ns2p6d10 configuration) has greater polarizing power than that with 8 elecvtron valence shell configuration (ns2p6 configuration). Example: Cu+-Cl- bond in CuCl is more covalent than Na+-Cl- bond in NaCl, since Cu+ has 3s2p6d10 configuration while Na+ has 2s2p6 configuration.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
The shapes of molecules can be predicted from the valence shell electron pair repulsion (VSEPR) theory. VSEPR theory proposes that the geometric arrangement of terminal atoms about a central atom in a covalent compound is determined solely by the repulsions between electron pairs present in the valence shell of the central atom. 
The basic assumptions of this theory are summarized below:
1) The electron pairs in the valence shell around the central atom of a molecule repel each other and tend to orient in space so as to minimize the repulsions and maximize the distance between them.
2) There are two types of valence shell electron pairs.
Bond pairs are shared by two atoms and are attracted by two nuclei. Hence they occupy less space and cause less repulsion. The bond pairs are usually represented by a solid line.
Lone pairs are not involved in bond formation and are in attraction with only one nucleus. Hence they occupy more space. As a result, the lone pairs cause more repulsion. The lone pairs are represented by a lobe with two electrons.
The order of repulsion between different types of electron pairs is as follows:
Lone pair-Lone pair > Lone Pair-Bond pair > Bond pair-Bond pair
3) The repulsion caused by bonds increases with increase in the number of bonded pairs between two atoms i.e., a triple bond causes more repulsion than a double bond which in turn causes more repulsion than a single bond.
4) The shape of a molecule can be predicted from the number and type of valence shell electron pairs around the central atom.
When the valence shell of central atom contains only bond pairs, the molecule assumes symmetrical geometry due to even repulsions between them. However the symmetry is distorted when there are also lone pairs along with bond pairs due to uneven repulsion forces.
Theory of covalent bond formation (Orbital Concept)
Valence bond theory (VBT)
In order to explain how a covalent bond is formed Heitler and London in 1927 put forward a theory which is called Valence Bond Theory (VBT).
The main postulates of Valence bond theory are as follows:
- A covalent bond is formed by the overlapping of two half-filled valence atomic orbitals of two different atoms.
- The overlap of two atomic orbitals gives rise to a single bond orbital which is a localised orbital and is occupied by both the electrons having opposite spins.
- The electrons in the overlapping orbitals get paired and confined between the nuclei of two atoms. The equilibrium distance at which the two atomic nuclei are now held is called the Bond length.
- The electron density between two bonded atoms increases due to overlapping. This confers stability to the molecule. Greater the extent of overlapping, stronger is the bond formed.
- The amount of energy given off or released per mole at the time of overlapping of atomic orbitals to form a bond is termed as Bond Energy or Stabilisation Energy.
Thus it is clear that, an atomic orbital of the outermost shell of an atom containing one electron has a tendency to overlap with another atomic orbital of another atom containing one electron of opposite spin. This type of overlap gives rise to the formation of a bond which is called covalent bond.
Types of overlapping and Nature of Covalent Bond
A covalent bond is of two types depending on the type of overlapping between the two atoms
(1) Sigma (σ) bond
(2) Pi (π) Bond
Sigma (σ) bond
A covalent bond which is formed between two atoms by the overlap of their half-filled atomic orbitals along the line joining the nuclei of both the atoms (i.e. along the nuclear axis, bond axis or molecular axis, as it is called) is called a σ-bond.
In other words, when there is end to end overlapping of half-filled atomic orbitals along the
inter-nuclear axis, the bond resulted is called sigma (σ) bond. This type of overlapping between the atomic orbitals is also called “head-on” overlapping or “axial” overlapping.
inter-nuclear axis, the bond resulted is called sigma (σ) bond. This type of overlapping between the atomic orbitals is also called “head-on” overlapping or “axial” overlapping.
It results when one of the following types of overlapping takes place:
(i) s-s overlap: In this type of overlap, half-filled s-orbital of one atom overlaps with the half-filled
s-orbital of the other atom. e.g. The formation of hydrogen molecule from two H-atoms.
s-orbital of the other atom. e.g. The formation of hydrogen molecule from two H-atoms.
(ii) s-p overlap: In this type of overlap, s-orbital of one atom overlaps with the half-filled p-orbital of the other atom. e.g Formation of hydrogen chloride molecule from H- and Cl-atoms.
(iii) p-p overlap: Here singly-filled p-orbital of one atom overlaps with the half-filled p-orbital of the other atom on internuclear axis atoms (nuclear axis, bond axis or molecular axis). e.g. The formation of Cl2 from two Cl-atoms.
Pi (π) Bond
A covalent bond which is formed between two atoms by the overlap of their singly-filled p-orbitals along a line perpendicular to their nuclear axis (side-to-side overlap) is called a π bond.
In other words it bond is produced by the side to-side overlap of half-filled p-orbitals of the two atoms. It is also called lateral or sidewise overlap.
Example: Formation of O2 , N2 molecule
This type of overlapping takes place perpendicular to the inter-nuclear axis as shown below:
Example: Formation of O2 , N2 molecule
This type of overlapping takes place perpendicular to the inter-nuclear axis as shown below:
p-p side-to-side overlap: This is also called
side-wise or side-way or lateral overlap. Here the overlap of two p-orbitals takes place along a line perpendicular to the molecular axis. Hence the name lateral overlap. This means that for this type of overlap the two p-orbitals must be held parallel to each other, i.e. the orbital aids should be parallel.
side-wise or side-way or lateral overlap. Here the overlap of two p-orbitals takes place along a line perpendicular to the molecular axis. Hence the name lateral overlap. This means that for this type of overlap the two p-orbitals must be held parallel to each other, i.e. the orbital aids should be parallel.
Strength of σ and π bonds
1. In the covalent bond formation, greater the extent of overlapping of orbitals, greater is the energy released, i.e. the higher will be the strength of the covalent bond. This implies that the strength of a covalent bond is proportional to the extent of overlapping between the atomic orbitals of participating atoms. During the formation of σ bond the extent of overlapping is more and hence a Sigma bond is stronger than Pi bond.
2. Electrons in the π bond are more diffused in a space i.e. more exposed to the environment than the electrons in a σ bond. Hence little energy is needed to break the π bond and thus weaker than the σ bond.
Differences between Sigma and Pi bonds
Sigma (σ) bond
|
Pi (π) Bond
|
1. It is formed by end to end overlapping of
half-filled atomic orbitals. |
1. It is formed by the sidewise overlapping of
half-filled p-orbitals only. |
2. Overlapping takes place along internuclear axis.
|
2. Overlapping takes place perpendicular tointernuclear axis.
|
3. The extent of overlapping is large and bond formed is stronger.
|
3. The extent of overlapping is small and bond formed is weaker.
|
4. The molecular orbital formed as a result of overlapping is symmetrical about the internuclear axis.
|
4. The molecular orbital formed as a result of overlapping consists of two lobes above and below the internuclear axis.
|
5. There is free rotation about σ bond and no geometrical isomers are possible.
|
5. There is no free rotation about π bond and geometrical isomers are possible.
|
6. Sigma bonds are formed on the internuclear axis, are able to rotate and are considered symmetrical.
|
6. In contrast to sigma bonds, pi bonds involve
p-orbitals and are not symmetrical around the axis and therefore cannot be rotated |
7. The bond can be present alone.
|
7. The bond is always formed in addition to sigma (σ) bond.
|
8. s and p orbitals can participate in the formation of σ bond
|
8. Only p-orbitals participate in the formation of π bond
|
Localized Bonding
Chemical bonds are formed when two nuclei share a pair of electrons between them. These bonds are considered combinations of overlapping one-electron atomic orbitals. The bond is strongest when the two electrons are confined to a region between the two nuclei. This type of bond is described as a localized bond. Orbitals with one electron overlap other orbitals also containing one electron of opposite spin to create an area of increased electron probability density or a chemical bond.
Resonance
According to resonance concept, if two (or more) alternate valence bond structures can be written for a molecule, the actual structure of the molecule is said to be a resonance of all these alternate structures. Example: H2 molecule resonates between the three structures (i), (ii) and (iii). H2 molecule is a resonance hybrid of these three structures.
Hybridization
The process of mixing pure atomic orbitals on an atom of nearly equal energy to produce a set of entirely new orbitals which are equal in number to the mixing orbitals, have the same energy and identical shapes and are symmetrically disposed in space round the atom is known as hybridization and the new orbitals produced are called hybrid orbitals. The process of hybridization is a hypothetical process.
Definition: Thus hybridization may precisely be defined as the phenomenon of mixing up
(or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new orbitals equal in number to the mixing orbitals and having same energy contents and identical shapes.
(or merging) of orbitals of an atom of nearly equal energy, giving rise to entirely new orbitals equal in number to the mixing orbitals and having same energy contents and identical shapes.
Possible hybridization schemes:
2nd row elements : sp sp2 sp3
3rd row elements also have : dsp3 d2sp3
RULES OF HYBRIDIZATION:
For hybridization to occur, it is necessary for the atom to satisfy the following conditions: |
(1) Orbitals on a single atom only would undergo hybridization.
|
(2) There should be very little difference of energy level between the orbitals mixing to form hybrid orbitals.
|
(3) Number of hybrid orbitals generated is equal to the number of hybridizing orbitals.
|
(4) The hybrid orbitals assume the direction of the dominating orbitals. For example, if s and p orbitals are to hybridize, the s orbital having no directional character, does not contribute towards the direction when p orbitals determine the directional character of the hybrid orbitals.
|
(5) It is the orbitals that undergo hybridization and not the electrons. For example, four orbitals of an oxygen atom (2 , 2 , 2 , 2 ) s2 2 1 1 p p p x y z belonging to second level (i.e., 2s, 2px, 2py, 2pz) can hybridize to give four hybrid orbitals, two of which have two electrons each (as before) and the other two have one electron each.
|
(6) The electron waves in hybrid orbitals repel each other and thus tend to be farthest apart.
|
Types of hybrid orbitals
Hybrid Orbital
|
Geometry of Molecule
|
Examples
| |
sp
|
Linear
|
BeCl2, HgCl2, HC≡CH
| |
sp2
|
Triangular Planar
|
BBr3, BF3, H2C=CH2,C6H6
| |
sp3
|
Tetrahedral
|
CH4, SiCl4, H3C–CH3
| |
sp2d
|
Square planar
|
[Cu(NH3)4]+2, Ni(CN)42–, XeF4
| |
sp3d
|
Trigonal bi-pyramidal
|
PCl5
| |
sp3d2
|
Octahedral
|
SF6, Co(NH3)6+3
| |
sp hybridization
The process by which an s and a p orbital mix and form two new equivalent hybrid orbitals is called sp hybridization. This hybrid orbital lie along a line and are , therefore, often referred to as Linear hybrid orbitals .Each sp orbital has 50%, s-character and 50% p-character. This gives an angle of 180º between the axes of the two orbitals.
Illustration: The Electronic Configuration of carbon in the ground state is 1s2 2s2 2p2. If energy is supplied to raise one of the 2s electrons to a higher energy level to fill the vacant 2p orbital, the electronic configuration of carbon in the excited state is
1s2 2s1 2px1 2py1 2pz1. Two sub-orbitals (i.e. 2s1 and 2px1) of C atom in the excited state mix together and redistribute their energy to give a set of two new equivalent orbitals which are known as sp hybrid orbitals.


1s2 2s1 2px1 2py1 2pz1. Two sub-orbitals (i.e. 2s1 and 2px1) of C atom in the excited state mix together and redistribute their energy to give a set of two new equivalent orbitals which are known as sp hybrid orbitals.
One of the 'sp' hybrid orbitals overlaps with the 'sp' hybrid orbital of the other carbon atom to form a sp-sp sigma bond. The remaining two sp hybrid orbitals of the two carbon atoms overlap with the s orbitals of two hydrogen atoms to form two sigma (sp-s) bonds.
The unhybridized 2py1 and 2pz1 orbitals of two carbon atoms overlap laterally to form two pi bonds. The combination of one (sp-sp) sigma bond and two (p-p) pi bonds gives rise to the triple bond of acetylene.
Formation of ethyne molecule. (a) Shows scheme of overlaps; (b) shows the bonds, σ bonds being indicated by straight lines; and (c) shows ball-and-stick model of ethyne (acetylene)
Thus ethyne molecule contains one σ and a two π bonds between the two carbons and each carbon is linked with one H-atom through σ bonds.
sp2 hybridization
When an s and two p orbitals mix up to hybridize, there result three new orbitals called sp2 hybrid orbitals (spoken as ‘sp two’). Each sp2 hybrid orbital has 33% s-character and 67% p-character.
They are directed at an angle 120º to one another & called Trigonal hybrids, the process being referred to as Trigonal hybridization.
They are directed at an angle 120º to one another & called Trigonal hybrids, the process being referred to as Trigonal hybridization.
Illustration: The Electronic Configuration of carbon in the ground state is 1s2 2s2 2p2.
If energy is supplied to raise one of the 2s electrons to a higher energy level to fill the vacant 2p orbital, the electronic configuration of carbon in the excited state is 1s2 2s1 2px1 2py1 2pz1. Three sub-orbitals
(i.e. 2s1 2px1 and 2py1) of C atom in the excited state mix together and redistribute their energy to give a set of three new equivalent orbitals which are known as sp2 hybrid orbitals. One of the 'sp2' hybrid orbitals overlaps with the 'sp2' hybrid orbital of the other carbon atom to form a sp2-sp2 sigma bond.
If energy is supplied to raise one of the 2s electrons to a higher energy level to fill the vacant 2p orbital, the electronic configuration of carbon in the excited state is 1s2 2s1 2px1 2py1 2pz1. Three sub-orbitals
(i.e. 2s1 2px1 and 2py1) of C atom in the excited state mix together and redistribute their energy to give a set of three new equivalent orbitals which are known as sp2 hybrid orbitals. One of the 'sp2' hybrid orbitals overlaps with the 'sp2' hybrid orbital of the other carbon atom to form a sp2-sp2 sigma bond.
The remaining four sp2 hybrid orbitals of the two carbon atoms overlap with the s orbitals of four hydrogen atoms to form four sigma (sp2-s) bonds. The unhybridized 2pz1 orbital of two carbon atoms overlap laterally to form a pi bonds. The combination of one (sp2-sp2) sigma bond and one (p-p) pi bonds gives rise to the double bond of ethene. 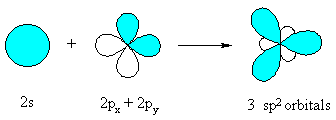
Orbital model of ethene molecule. ( a) shows scheme of overlaps; ( b) shows the bonds, sigma bonds indicated by straight lines; and ( c) shows ball-and-stick model of ethene (ethylene).
Thus the carbon to carbon double bond in ethene is made of one σ bond and one π bond.
sp3 hybridization
The Electronic Configuration of carbon in the ground state is 1s2 2s2 2p2. If energy is supplied to raise one of the 2s electrons to a higher energy level to fill the vacant 2p orbital, the electronic configuration of carbon in the excited state is 1s2 2s1 2px1 2py1 2pz1. Four sub-orbitals (i.e. 2s1 2px1 2py1 and 2pz1) of C atom in the excited state mix together and redistribute their energy to give a set of four new equivalent orbitals which are known as sp3 hybrid orbitals.
The bonds between the hydrogen atoms & carbon of methane are formed by the end-on overlap of these sp3 hybrids orbitals to form sigma bonds. These new orbitals are arranged as far apart in space as possible. Thus, the new orbitals point to the corners of a regular tetrahedron and the angle between any two hybrid orbitals 109.5 degrees.
Limitations of Valence Bond Theory
Though the valence bond theory explains the geometry of many molecules and ions yet it has following limitations:
(1) The presence of other nuclei should affect the electronic arrangement of all the atoms in the molecule
(2) Sometimes a single electronic structure does not explain all known properties of that molecule or ion and we have the many electronic structures called resonating structures.
(3) Valence Bond theory fails to explain the bonding in electron deficient compounds.
(4) It fails to explain the paramagnetic character of oxygen molecule.
Molecular Orbital Theory (MOT) of Covalent Bond
Theory: According to MOT, put forward by Hund and Muflikan all the atomic orbitals (AOs) of the atoms participating in the formation of the molecule approach nearer to each other and get mixed up to give an equivalent number of new orbitals that now belong to the molecule as a whole. These new orbitals are called molecular orbitals (MOs).
Features
The main features of molecular orbital theory can be summed up as follows:
The main features of molecular orbital theory can be summed up as follows:
(1) A molecule is quite different from its constituent atoms. All the electrons belong to the constituent atom and are considered to be moving under the influence of all nuclei.
(2) Atomic orbitals of individual atoms combine to form molecular orbitals and these MOs are filled up in the same way as atomic orbitals are formed. In other words, Pauli’s exclusion principle, Aufbau principle and Hund’s rule of maximum multiplicity are followed.
(3) The molecular orbitals have definite energy levels.
(4) The shapes of MOs formed depend upon the shape of combining atomic orbitals.
Linear Combination of Atomic orbitals (LCAO method)
According to this method, the molecular orbitals are formed by the linear combination (addition or subtraction) of atomic orbitals of the constituent atoms of the molecule.
Let us consider the simplest case of H2 molecule consisting of two hydrogen atoms represented by HA and HB. The atomic orbitals of these atoms are represented by the wave functions ψA and ψB. When these atoms approach each other there come two possibilities.
(1) Molecular orbital is formed by the addition of wave functions of atomic orbitals. It can be represented by ψ(MO) = ψA + ψB ……………..(i)
The M.O. formed is called bonding molecular orbital. It lowers the energy and brings about the stability in the system.
(2) Molecular orbital is formed by the subtraction of wave functions of atomic orbitals. It can be represented by ψ*(MO) = ψA – ψB …………….(ii)
The MO formed is called antibonding molecular orbital. This type of MO corresponds to higher energy state. It has net disruptive effect. That is why this MO is termed as antibonding molecular orbital, distinguished by attaching an asterisk (*) mark with the symbolic name of the molecular orbital. The molecular orbitals formed by the combination of 1s orbitals of two hydrogen atoms is shown below:
The molecular orbital obtained by addition (+ +) overlap has lower energy than either of the two 1s orbitals and hence is called bonding molecular orbital. It does not show any change of sign on rotation through 1800 through the inter-nuclear axis.
The molecular orbital obtained by subtraction (+ -) overlap is of higher energy than either of the two 1s-orbitals and hence is called antibonding molecular orbital. The resulting molecular orbital has zero electron density (nodal plane) in the region between the two nuclei (shown by solid line).
Relative Energies of bonding and antibonding MOs
We have seen above that when atomic orbitals combine, an equivalent number of new orbitals is formed. For example, when two 1s orbitals combine two new molecular orbitals are formed. One of these pertains to the bonding molecular orbital with lower energy while the other corresponds to higher energy as compared to both the atomic orbitals concerned in the process. Thus we find that the number of molecular orbitals formed from atomic orbitals is equal to the number of atomic orbitals responsible for their formation and are of two types as shown below.
Normally, the two electrons in hydrogen occupy the bonding molecular orbital, with anti-parallel spins. If molecular hydrogen is irradiated by ultra-violet (UV) light, the molecule may absorb the energy, and promote one electron into its anti-bonding orbital (σ*), and the atoms will separate. The energy levels in a hydrogen molecule can be represented in a diagram - showing how the two 1s atomic orbitals combine to form two molecular orbitals, one bonding (σ) and one anti-bonding (σ *).
3 Co-ordinate bond/Dative bond
(Bonding by One-sided Sharing of Electrons or Bonding by Electron Pair Donation)
(Bonding by One-sided Sharing of Electrons or Bonding by Electron Pair Donation)
In a normal covalent bond, each of the two bonded atoms contributes one electron to make the shared pair. In some cases, a covalent bond is formed when both the electrons are supplied entirely by one atom. Such a bond is called co-ordinate covalent or dative bond.
Definition: A covalent bond which is formed by mutual sharing of two electrons both of which are provided entirely by one of the linked atoms or ions is called co-ordinate bond.
Definition: A covalent bond which is formed by mutual sharing of two electrons both of which are provided entirely by one of the linked atoms or ions is called co-ordinate bond.
Co-ordinate bond is also sometimes referred to as co-ordinate covalent bond or dative bond.
The compounds containing a coordinate bond are called coordinate compounds. The pair of shared electrons is called lone pair. The atom which furnishes the electron pair is called donor or ligand while the other atom which accepts the electron pair is called acceptor. A coordinate bond is represented by an arrow which points away from the donor to the acceptor.
The compounds containing a coordinate bond are called coordinate compounds. The pair of shared electrons is called lone pair. The atom which furnishes the electron pair is called donor or ligand while the other atom which accepts the electron pair is called acceptor. A coordinate bond is represented by an arrow which points away from the donor to the acceptor.
e.g. H2O2, O3, SO2, SO3 Molecules
Illustration of the formation of a co-ordinate bond
The donor atom A has a lone pair of electrons on it, while the acceptor atom B is short of two electrons than the octet in its valence shell. A donates its lone pair to B which accepts it. Thus the two electrons of the lone pair from A are now shared by both the atoms and this mutual sharing of electron pair results in the formation of a co-ordinate bond between A and B (A → B).
Conditions for the formation of co-ordinate bond
- The donor atom should have a lone pair of electrons.
- The acceptor atom should have a vacant orbital.
Properties of co-ordinate compounds
- Melting and boiling points: Ionic compounds > co-ordinate compounds > covalent compounds
- Semi polar character: Ionic compounds > co-ordinate compounds > covalent compounds
- Physical state: Gas, liquid or solid.
- Solubility: Soluble in organic solvents.
- Conductivity: They do not conduct electric current in aqueous solution or in molten state.
- Isomerism: Like covalent compounds co-ordinate compounds also show isomerism.
4 Metallic bond
The metal atoms in a metal crystal are bonded together by a bond which is neither an ionic bond nor a covalent bond, but it is a special type of bond which is called metallic bond.
Definition: The particular type of bonding which holds the metal atoms together in a metal crystal is called metallic bonding.
Nature of metallic bond: In metals, the metal atoms lose their outer electrons to form metal cations. The electrons from all the metal atoms form a "sea" of electrons that can flow around these metal cations. These electrons are often described as delocalised electrons (not fixed in one place or free to move). As the metal cations and the electrons are oppositely charged, they will be attracted to each other, and also to other metal cations. These electrostatic forces are called metallic bonds, and these are what hold the particles together in metals.
THE ELECTRON SEA MODEL : A metal is regarded as a group of positive metal ions packed together as closely as possible in a regular geometric pattern and immersed in a sea of electrons (called electron-pool or electron-gas or electron-cloud) which move about freely (mobile or delocalised electrons) in the vacant valence orbitals. The attractive force that binds the metal ions to the mobile electrons is called metallic bond and the force of attraction between the metal ions and the mobile electrons holds the atoms together.
① Hydrogen bond
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule.
The attractive electrostatic force between a hydrogen atom which is already covalently attached with a strongly electronegative atom of a molecule and another electronegative atom of some other molecule (same molecule or different molecule) is known as hydrogen bond.
Example: H2O with other H2O, NH3 with other NH3
Illustration of the formation of a hydrogen bond
In order to understand the concept of hydrogen bond let us consider a molecule, say, AH in which H atom is linked with a strongly electronegative but very small atom X (X may be N, O or F) by a normal covalent bond. Since the electronegative atom, X attracts the electron pair towards itself, thus will have a lone pair of electrons. This leaves H atom with a large partial positive charge. Consequently AH molecule will behave as a dipole which is represented as: 
Evidently the dipole has X as its negative end and H as its positive end.
Now if another molecule like XH (same molecule) or YH (different molecule) (X and Y are strongly electronegative atoms) which also forms a dipole X - or Y - H respectively is brought near A - dipole, these two dipoles will be attracted towards each other by electrostatic force of attraction which is represented by a dotted or dashed line and is called hydrogen bond or hydrogen bonding.
The hydrogen must be attached to a strongly electronegative heteroatom, such as oxygen, nitrogen or fluorine, which is called the hydrogen bond donor. A hydrogen bond results when this strong positive charge density attracts a lone pair of electrons on another heteroatom, which becomes the hydrogen bond acceptor.
Types of hydrogen bond
- Inter-molecular hydrogen bond: This type of hydrogen bond occurs between two or more molecules of the same or different compound. e.g.: NH3, H2O etc.
- Intra-molecular hydrogen bond
This type of hydrogen bond is formed between a hydrogen atom and an electronegative atom present in the same molecules. O-hydroxy benzaldehyde→
Example: o-nitrophenol, 2-nitrobenzoic acid etc.
Example: o-nitrophenol, 2-nitrobenzoic acid etc.
Significance of hydrogen bonding
1. Physical state of water: Without hydrogen bonding, H2O would have existed as a gas like H2S. In that case no life would have been possible.
2. Applications in biological investigations: Hydrogen bonding also exists in molecules of living system like various tissues, organs, skin in animals. The molecular conformation of protein, nucleic acid etc. are determined by hydrogen bond.
Protein: Hydrogen bonding plays an important role in determining the three-dimensional structures adopted by proteins. In these macromolecules, bonding between parts of the same macromolecule cause it to fold into a specific shape, which helps determine the molecule's physiological or biochemical role.
The breakdown of protein into amino acid is due to the weak hydrogen bond. The NH and CO vertically adjacent to each other in the helix are linked together by H- bond.
DNA: The double helical structure of DNA, for example, is due largely to hydrogen bonding between the base pairs, which link one complementary strand to the other and enable replication.
Drug action: Hydrogen bonding increases the activity of drug. For example, (–) ephedrine is more active than (+) ephedrine since (–) ephedrine forms hydrogen bonding with biological receptor.
②Van der Waals force
Van der Waals forces are very short lived intermolecular attractive forces which are believed to exist between all kinds of atoms, molecules and ions when they are sufficiently close to each other.
Types of van der Waals forces:
1. Dipole-dipole interaction (Keesom Forces): These forces are found in polar molecules having permanent polarity in them. When polar molecules are brought nearer to each other, they orient themselves in such a way that the positive end of the one dipole attracts the negative end of another dipole and vice versa. This is the strongest of all other types of van der Waals forces. 
2. Ion-dipole interaction
When NaCl is put in H2O, it dissolves in it, since the negative ends of water molecule dipoles aggregate around Na+ ions and the positive ends around Cl- ions.
3. Dipole-induced dipole interaction (Debye Forces)
This type of force is found in a mixture containing polar and nonpolar molecules. When a nonpolar molecule is brought near to a polar molecule, the positive end of the polar molecule attracts the mobile electrons of the non-polar molecule and thus polarity is induced in nonpolar molecule. Now both the molecules become dipoles and hence the positive end of the polar molecule attracts the displaced electron cloud of nonpolar molecule.
4. Instantaneous dipole-induced dipole interaction (London Forces)
A nonpolar atom or molecule may be visualized as a positive center surrounded by asymmetrical negative electron cloud. But as the electron cloud oscillates, the electron cloud becomes denser on one side of the molecule than on the other side. The displacement of electron cloud creates an instantaneous dipole temporarily. Thus the non-polar molecule becomes temporarily polar. Now when the self-polarized molecule is brought near to a non-polar molecule, it polarizes the neighboring molecule by disturbing its electronic distribution and thus an induced dipole is created. These temporarily polar molecules are mutually attracted by weak attractive forces.
The End